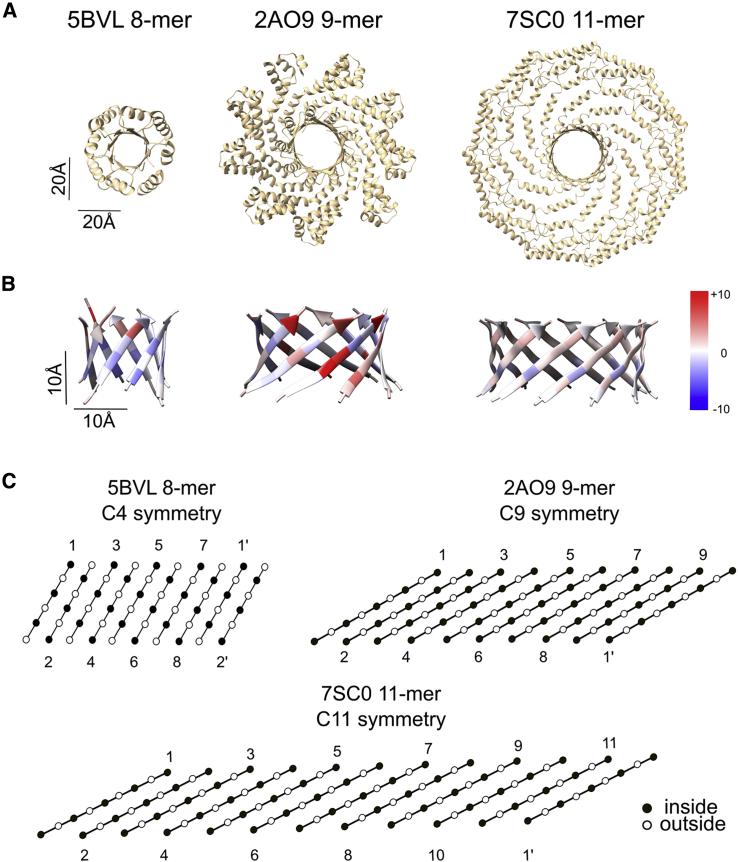
Figure 7.
Comparison of structural features of CAV1 β-barrel with other parallel β-barrels. (A) The full structures of the assemblies from which the analyzed parallel β-barrels were taken viewed in the direction of the barrel pore. All structures are shown to scale. (B) Structures and the per-residue energy breakdowns of three parallel β-barrels calculated with Rosetta. Left panel, a representative TIM barrel with C4 symmetry (PDB: 5BVL); middle panel, a phage protein from Bacillus cereus with C9 symmetry (PDB: 2AO9); and right panel, experimentally determined CAV1 β-barrel with C11 symmetry (PDB: 7SC0). Red indicates positive scores (destabilizing), and blue indicates negative scores (stabilizing). (C) Amino acid alignments of the 8-, 9-, and 11-meric β-barrel structures. Black circles indicate pore-facing residues, and white circles indicate residues facing away from the pore. Individual strands are labeled. Primes denote the same strand as the starting strand and are included to show the relative levels of the first and last strands in the barrel.