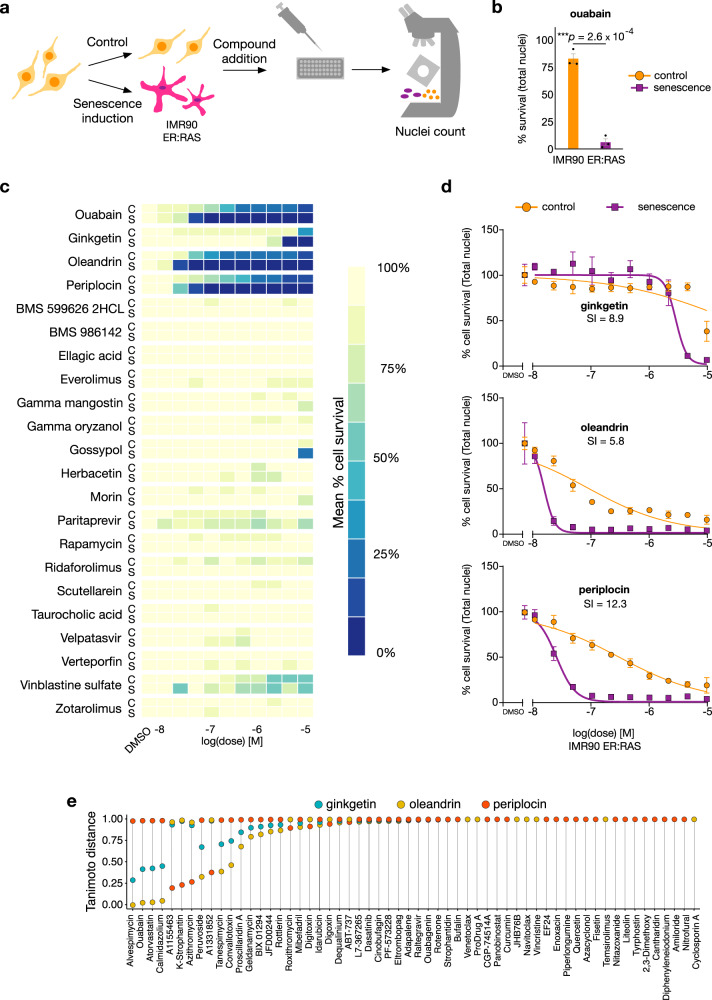
Fig. 3. Experimental characterisation of compounds selected for screening in oncogene-induced senescent (OIS) cells.
a Experimental setup of OIS model with IMR90 ER:RAS cells. Senescence was induced by addition of 4-OHT at 100 nM during the duration of the experiment (8 days). Control and senescent cells were plated in a 384-well plate on day five of 4-OHT induction. Top predicted compounds were added after multiwell seeding, and 72 h afterwards, the cells were fixed, and the nuclei stained and counted. b Bar plot of OIS positive experimental control, ouabain, at 46.4 nM. Data is normalised to DMSO. Data represented as individual points, and bars and error bars represent the mean ± SEM of three independent experiments. Statistical analysis was performed using a two-sided two-sample t-test for difference in mean value: ***p < 0.001; p = 2.6 × 10−4. c Results from experimental validation of controls and the top 21 compounds from Fig. 2d predicted to have senolytic action with P > 44%. Three compounds out of the 21 displayed senolytic activity: ginkgetin, oleandrin and periplocin; heatmap shows mean across n = 3 replicates. This drug screen was done once with three experimental replicates. d Dose-response curves of the three newly found senolytic compounds. The senolytic index (SI) is defined as the ratio between the IC50 of control cells and the IC50 of senescent cells. Data is normalised to DMSO. Mean ± s.d. are shown from n = 3 experiments. Oleandrin and periplocin are related steroid saponins, similar to ouabain. Ginkgetin is a structurally distinct biflavone; the structures of the three compounds can be found in Supplementary Fig. 11. e Tanimoto distance between the three validated senolytics and those employed for model training; distances were calculated using the RDKit descriptors that were employed in the training of machine learning models in Fig. 2b and Supplementary Table 2. Source data are provided as a Source Data file.