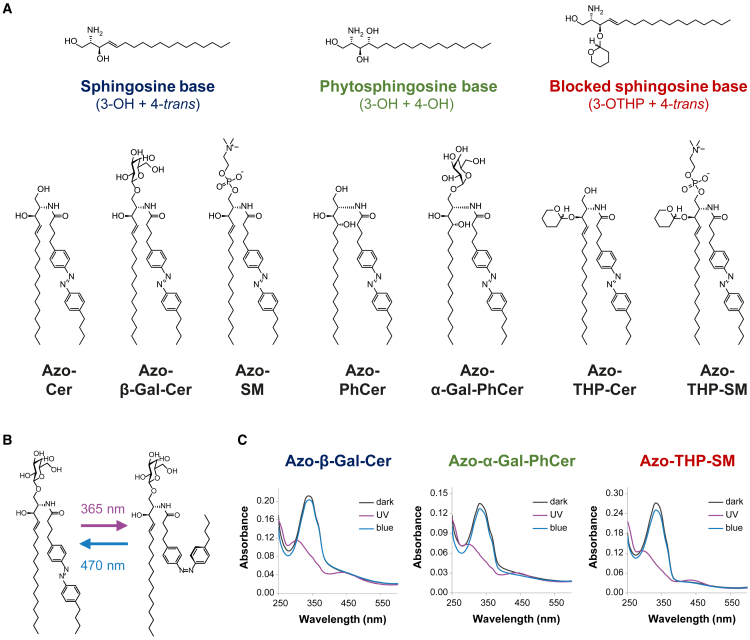
Figure 1.
Structural and spectral properties of photoswitchable azo-sphingolipids. (A) Chemical structures of the N-acyl azobenzene-modified (FAazo-4 fatty acid) sphingolipids here tested, subdivided according to their sphingoid backbone: Azo-Cer, Azo-β-Gal-Cer, and Azo-SM with a sphingosine base and Azo-PhCer and Azo-α-Gal-PhCer with a phytosphingosine base, as well as Azo-THP-Cer and Azo-THP-SM displaying a 3-OH-blocked sphingosine base with a THP protecting group. (B) Schematics of light-induced trans-cis isomerization for an azo-sphingolipid, notably Azo-β-Gal-Cer. Application of UV-A light (λ = 365 nm) leads to the formation of a cis-photolipid, while illumination with blue light (λ = 470 nm) leads to the formation of a trans-photolipid. (C) UV-vis absorbance spectra of photoswitchable sphingolipids (notably Azo-β-Gal-Cer, Azo-α-Gal-PhCer, and Azo-THP-SM) incorporated in SUVs at the dark-adapted state (black curves), as well as after the sequential shining of UV-A (purple curves) and blue light (blue curves). To see this figure in color, go online.