Abstract
Background
Intrathecal baclofen therapy can substantially improve symptoms in most patients with severe spasticity due to traumatic spinal cord injury, multiple sclerosis, or cerebral paresis. To the best of our knowledge, decompression surgeries at the intrathecal catheter insertion site in patients with a preexisting intrathecal pump for drug delivery have not been reported.
Case presentation
We report the case of a 61-year-old Japanese man with lumbar spinal stenosis who underwent intrathecal baclofen therapy. We performed decompression for lumbar spinal stenosis at the intrathecal catheter insertion site during intrathecal baclofen therapy. The yellow ligament was removed by partial resection of the lamina under a microscope to avoid damage to the intrathecal catheter. The dura mater was distended. No obvious cerebrospinal fluid leakage was observed. Postoperatively, lumbar spinal stenosis symptoms improved, and spasticity remained well controlled with intrathecal baclofen therapy.
Conclusions
This is the first reported case of lumbar spinal stenosis decompression at an intrathecal catheter insertion site during intrathecal baclofen therapy. Preoperative preparation is necessary, as the intrathecal catheter may be replaced during surgery. We performed surgery without removing or replacing the intrathecal catheter, taking care not to damage the spinal cord by migrating the intrathecal catheter.
Keywords: Baclofen, Intrathecal catheter, Lumbar spinal stenosis, Decompression
Background
To the best of our knowledge, decompression surgeries at the intrathecal catheter insertion site in patients with a preexisting intrathecal pump for drug delivery have not been reported. We report a case of decompression for lumbar spinal stenosis (LSS) at the intrathecal catheter insertion site during intrathecal baclofen (ITB) therapy.
Case presentation
A 61-year-old Japanese man had a cervical spinal cord injury approximately 2 years previously, and underwent cervical laminoplasty from C4 to C7 at a different hospital. Preoperative magnetic resonance imaging (MRI) revealed spinal canal stenosis at C5/6 and C6/7, and MRI after laminoplasty revealed decompression of the spinal cord (Fig. 1). He was referred to our hospital 6 months after surgery because of progressively worsening spasticity of the lower limbs. We performed ITB pump (SynchroMed II, Medtronic, Inc., Minneapolis, MN, USA) implantation by inserting an intrathecal catheter through the L2/3 interlaminar space to the T8/9 level (Fig. 2). After surgery, his spastic gait improved and progressed well; however, 1 year after surgery, intermittent claudication was observed, making it difficult for the patient to walk long distances. Physical examination revealed numbness and mild muscle weakness in both lower extremities. No signs of spinal tension were observed. MRI of the lumbar spine revealed multiple LSS at L1/2, L2/3, L3/4, and L4/5 (Fig. 3). Based on these findings, we planned to decompress all stenotic areas. We also considered the possibility that the intrathecal catheter inserted through the L2/3 interlaminar space would need to be removed in preparation for surgery.
Fig. 1.
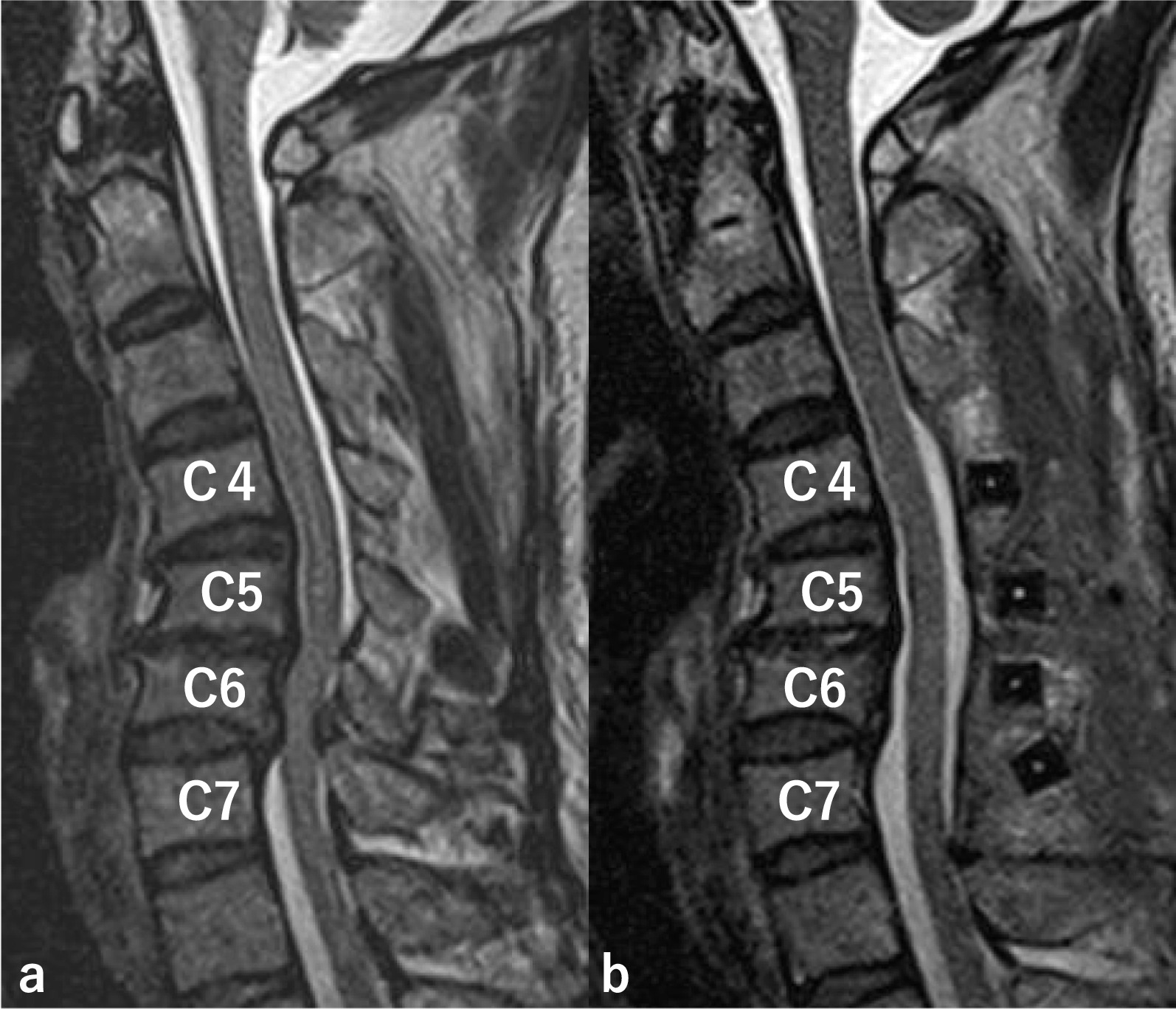
Preoperative magnetic resonance imaging (MRI) and MRI after laminoplasty. Preoperative MRI shows spinal canal stenosis at C5/6 and C6/7, and MRI after laminoplasty reveals decompression of the spinal cord. a Preoperative MRI, b MRI after laminoplasty
Fig. 2.
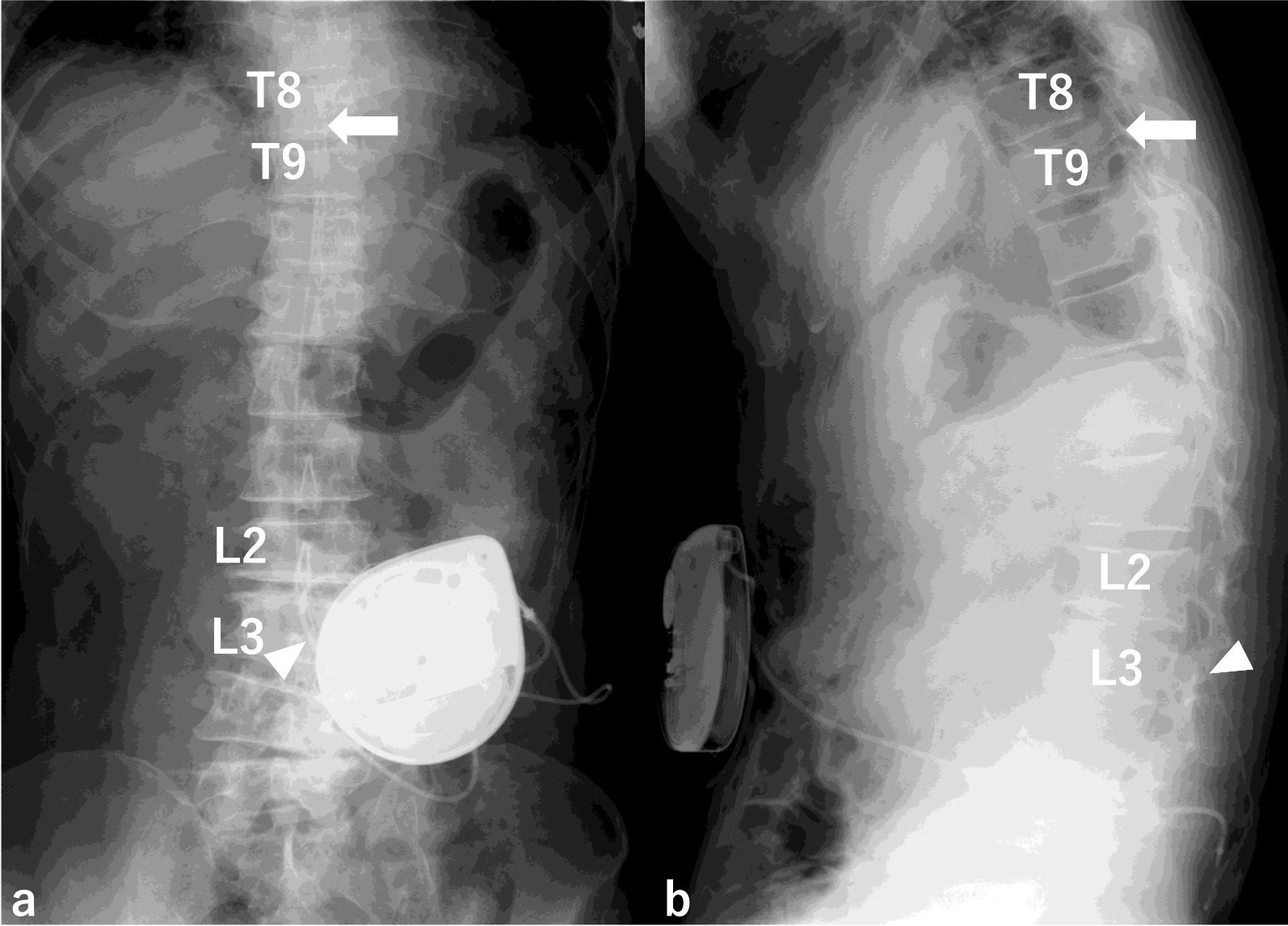
Intrathecal baclofen (ITB) pump implantation. ITB pump implantation was performed by inserting the intrathecal catheter through L2/3 interspace (white solid triangle) to T8/9 level (white solid arrow). a AP view b Lateral view
Fig. 3.
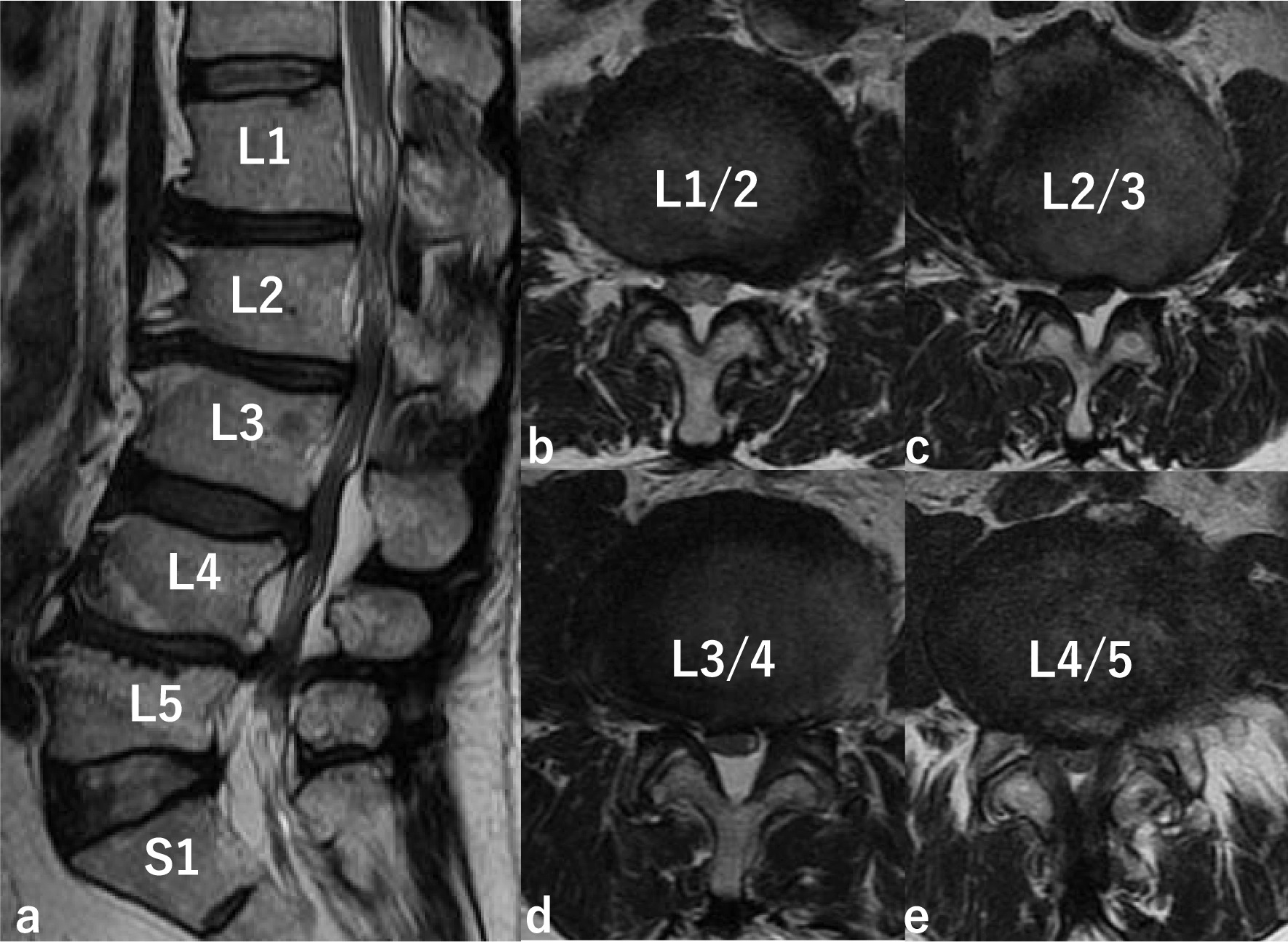
MRI of the lumbar spine. MRI shows multiple lumbar spinal stenosis (LSS) on T2-weighted imaging (a sagittal; b L1/2 axial; c L2/3 axial; d L3/4 axial; e L4/5 axial)
Results
Electrocautery was not used due to the risk of impaired pump operability. As we deployed the subcutaneous tissue, we found the connector pin and sleeve connecting the pump segment of the intrathecal catheter to the spinal segment and the anchor securing the spinal segment of the intrathecal catheter. Once we opened the lumbar lamina, we observed the spinal segment of the intrathecal catheter inserted through the L2/3 interlaminar space. The intrathecal catheter and L2 lamina proximal to the intrathecal catheter was confirmed under a microscope. Partial resection of the L2 lamina was performed with a high-speed diamond-tipped burr with a diameter of 4 mm under a microscope. The intrathecal catheter was then retracted with a nerve root retractor, and resection of the ligamentum flavum and partial resection of L3 lamina were performed with the Kerrison rongeur under a microscope, so as not to damage the intrathecal catheter. When retracting the intrathecal catheter with a nerve root retractor, the retraction was kept to a minimum so as not to damage the spinal cord at the tip of the intrathecal catheter. The dura mater was distended, and no obvious cerebrospinal fluid leakage was observed (Fig. 4).
Fig. 4.
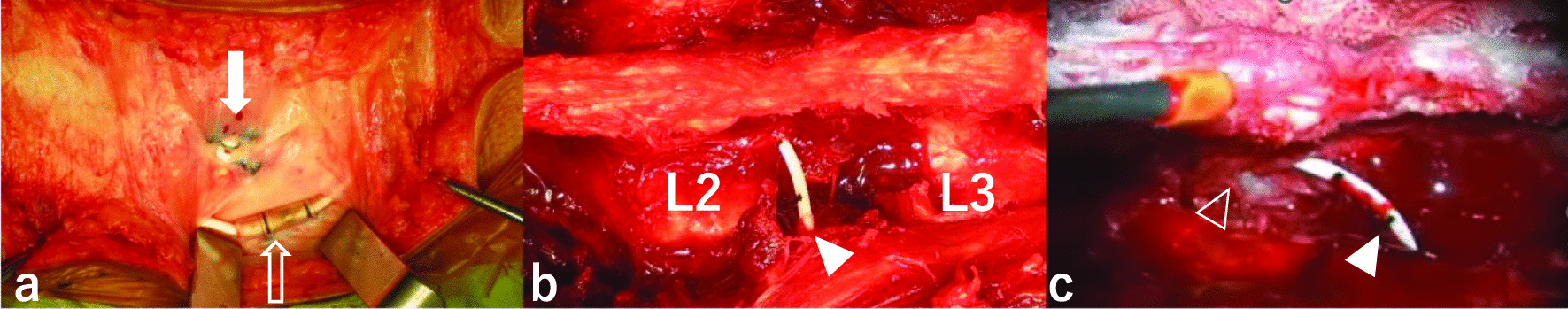
Intraoperative findings. The connector pin and sleeve (white outline arrow) connecting the intrathecal catheter’s pump segment to the spinal segment and anchor (white solid arrow) securing the spinal segment of the intrathecal catheter. The spinal segment of the intrathecal catheter (white solid triangle) through L2/3 interlaminar space. The dura mater (white outline triangle) is distended. a superficial layer b deep layer c post decompression
Postoperative three-dimensional computed tomography confirmed that the intrathecal catheter was inserted through the L2/3 interlaminar space to the original position at the T8/9 level without any damage to the intrathecal catheter and the LSS was decompressed (Fig. 5). Postoperatively, the patient’s gait was good, and although his spasticity persisted, he progressed well with ITB therapy. The pain in the left lower extremity recurred 8 months after surgery. We diagnosed the patient with neurological symptoms of lumbar foraminal stenosis with associated scoliosis. Additional posterior lumbar interbody fusion (PLIF) was performed at L4/5 and L5/S. Deployment was performed as previously described. The spinal segment of the intrathecal catheter could not be identified because the surgical site was distal to the intrathecal catheter insertion site. X-ray examination after PLIF revealed that the intrathecal catheter was inserted through the L2/3 interlaminar space to the original T8/9 level (Fig. 6). Additional lumbar fusion surgery improved the gait, and 9 years and 5 months after lumbar fusion surgery spasticity continued to be well controlled with ITB therapy. We performed both operations without removing or replacing the intrathecal catheter, taking care not to damage the intrathecal catheter or spinal cord.
Fig. 5.
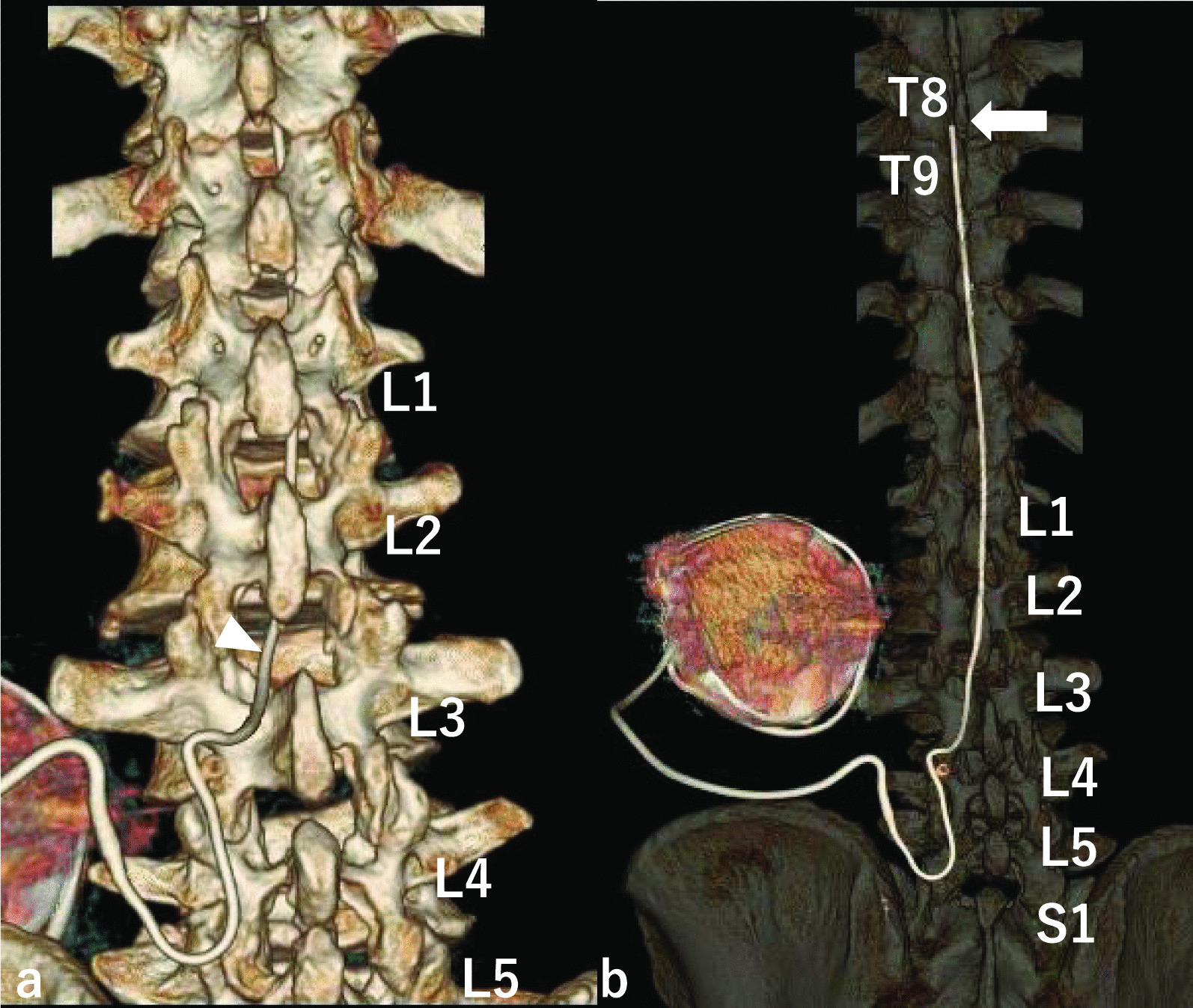
Postoperative three-dimensional computed tomography (3D-CT) scan. Postoperative 3D-CT scan confirms that the intrathecal catheter was inserted through the L2/3 interlaminar space to the original position T8/9 level without damaging the intrathecal catheter, and lumbar spinal stenosis (LSS) was decompressed. White solid arrow: the tip of the intrathecal catheter. White solid triangle: the insertion site of the intrathecal catheter. a 3D-CT b 3D-CT Easier to see the intrathecal catheter
Fig. 6.
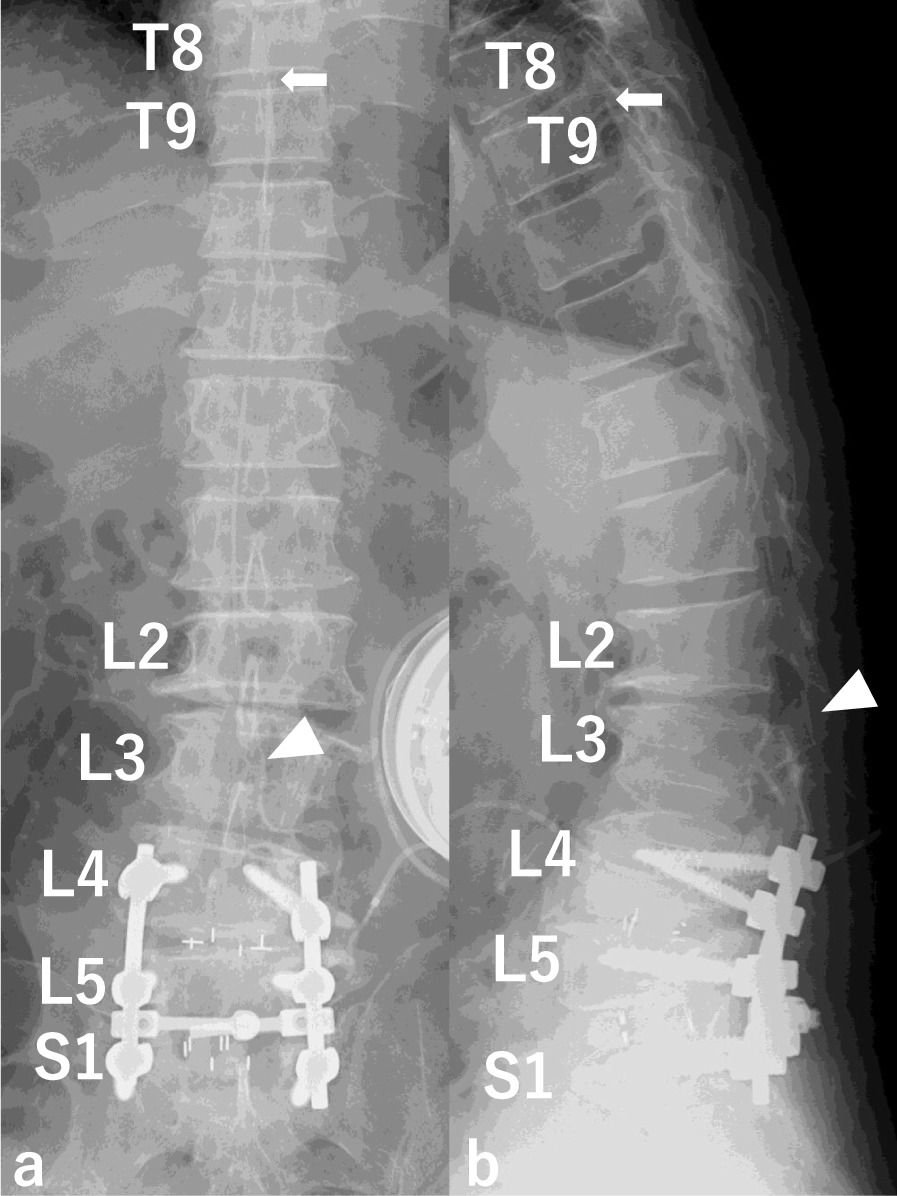
Postoperative X-ray. The intrathecal catheter is inserted through the L2/3 interlaminar space to the original position T8/9 level. White solid triangle: the insertion site of the intrathecal catheter. a AP view b Lateral view
Discussion and conclusions
ITB effectively improves spasticity in patients whose spasticity is not sufficiently managed by oral baclofen or other oral antispastic medications. Baclofen withdrawal syndrome is a potentially life-threatening complication [1–8].
To the best of our knowledge, decompression surgeries at the intrathecal catheter insertion site in patients with a preexisting intrathecal pump for drug delivery have not been reported. There are only two reports of posterior spinal fusion in which baclofen was not delivered despite a normal functioning pump [9, 10]. Alden et al. [9] reported a case of posterior spinal fusion for scoliosis in which baclofen was not delivered despite the use of a normal functioning pump. The report offered no details concerning the type of surgery or possible cause of intrathecal catheter malfunction. Fernandes et al. [10] reported a case of posterior spinal fusion for neuromuscular scoliosis that developed ITB withdrawal syndrome, likely caused by a small nick created in the intrathecal catheter tubing during dissection. The authors described three available surgical methods to prevent this complication. First, the intrathecal catheter was removed during the exposure and reintroduced at the end of the procedure. Second, intrathecal catheter preservation can be attempted through delicate dissection and isolation. Third, the intrathecal catheter was sectioned and reattached at the end of the procedure using a connector. The first option is related to low-pressure headaches secondary to presumed cerebrospinal fluid leakage. The second option has potential complications during the procedure: catheter injury may occur at various points, and the intrathecal catheter can be pulled out to a level distal to the intended level or from the spinal canal itself. The third option must be coordinated with the neurosurgical service to ensure that the pump catheter system functions properly afterwards.
We report the first case of decompression for LSS at an intrathecal catheter insertion site during ITB therapy. Preoperative preparation is necessary as the intrathecal catheter may be replaced during surgery. Care was taken not to damage the intrathecal catheter or spinal cord, and treatment was possible without removing or replacing the intrathecal catheter. Surgeons must be aware of ITB withdrawal syndrome and develop strategies to prevent interruption of baclofen delivery.
This is the first report of decompression of LSS at an intrathecal catheter insertion site during ITB therapy. Preoperative preparation is necessary as the intrathecal catheter may be replaced during surgery. Care was taken not to damage the intrathecal catheter or spinal cord, and treatment was possible without removing or replacing the intrathecal catheter.
Acknowledgements
Not applicable. No assistance was provided in writing the manuscript.
Abbreviations
- ITB
Intrathecal baclofen
- LSS
Lumbar spinal stenosis
- MRI
Magnetic resonance imaging
- PLIF
Posterior lumbar interbody fusion
Author contributions
YT performed the surgery. YT, HY, HE, HH, HI, KT, AM, YK, AN, KK, TY, and HT designed the treatment plans. YT conducted the follow-up. YT wrote the manuscript draft, which was revised by HT. All the authors have read and approved the final manuscript.
Funding
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest or financial conflict with the subject matter or materials discussed in this manuscript.
Availability of data and materials
Medical imaging data will not be shared because it is not fully anonymous.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent form is available for review by the Editor-in-Chief of the journal.
Competing interests
The authors declare that they have no competing interests pertaining to this manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yasutaka Takagi, Email: takagi@p1.coralnet.or.jp.
Hiroshi Yamada, Email: hiroshiy@v004.vaio.ne.jp.
Hidehumi Ebara, Email: ebapyon@yahoo.co.jp.
Hiroyuki Hayashi, Email: snoopeanuts27@hotmail.com.
Hiroyuki Inatani, Email: i.hiroyuki69@gmail.com.
Kazu Toyooka, Email: kazusj17@gmail.com.
Akari Mori, Email: 3tlxt45@gmail.com.
Yoshiyuki Kitano, Email: kibitaki@beige.plala.or.jp.
Aki Nakanami, Email: akichan.4@nifty.com.
Kenji Kagechika, Email: kagetika@p1.coralnet.or.jp.
Tetsutaro Yahata, Email: yahata@med.kanazawa-u.ac.jp.
Hiroyuki Tsuchiya, Email: tsuchi@med.kanazawa-u.ac.jp.
References
- 1.Terrence CF, Fromm GH. Complications of Baclofen withdrawal. Arch Neurol. 1981;38:588–589. doi: 10.1001/archneur.1981.00510090082011. [DOI] [PubMed] [Google Scholar]
- 2.Sampathkumar P, Scanlon PD, Plevak DJ. Baclofen withdrawal presenting as multiorgan system failure. Anesth Analg. 1998;87:562–563. doi: 10.1097/00000539-199809000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Green LB, Nelson VS. Death after acute withdrawal of intrathecal Baclofen: case report and literature review. Arch Phys Med Rehabil. 1999;80:1600–1604. doi: 10.1016/s0003-9993(99)90337-4. [DOI] [PubMed] [Google Scholar]
- 4.Coffey RJ, Edgar TS, Francisco GE, Graziani V, Meythaler JM, Ridgely PM, et al. Abrupt withdrawal from intrathecal Baclofen: recognition and management of a potentially life-threatening syndrome. Arch Phys Med Rehabil. 2002;83:735–741. doi: 10.1053/apmr.2002.32820. [DOI] [PubMed] [Google Scholar]
- 5.Mohammed I, Hussain A. Intrathecal Baclofen withdrawal syndrome—a life-threatening complication of Baclofen pump: a case report. BMC Clin Pharmacol. 2004;4:6. doi: 10.1186/1472-6904-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watve SV, Sivan M, Raza WA, Jamil FF. Management of acute overdose or withdrawal state in intrathecal Baclofen therapy. Spinal Cord. 2012;50:107–111. doi: 10.1038/sc.2011.112. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso AL, Quintaneiro C, Seabra H, Teixeira C. Cardiac arrest due to Baclofen withdrawal syndrome. BMJ Case Rep. 2014;2014:bcr2014204322. doi: 10.1136/bcr-2014-204322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romito JW, Turner ER, Rosener JA, Coldiron L, Udipi A, Nohrn L, et al. Baclofen therapeutics, toxicity, and withdrawal: a narrative review. SAGE Open Med. 2021;9:20503121211022197. doi: 10.1177/20503121211022197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alden TD, Lytle RA, Park TS, Noetzel MJ, Ojemann JG. Intrathecal Baclofen withdrawal: a case report and review of the literature. Childs Nerv Syst. 2002;18:522–525. doi: 10.1007/s00381-002-0634-8. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes P, Dolan L, Weinstein SL. Intrathecal Baclofen withdrawal syndrome following posterior spinal fusion for neuromuscular scoliosis: a case report. Iowa Orthop J. 2008;28:77–80. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Medical imaging data will not be shared because it is not fully anonymous.


