Abstract
Background:
Whether initiation of statins could increase survival free of dementia and disability in adults aged ≥75 years is unknown.
Methods:
PREVENTABLE, a double-blind, placebo-controlled randomized pragmatic clinical trial, will compare high-intensity statin therapy (atorvastatin 40 mg) with placebo in 20,000 community-dwelling adults aged ≥75 years without cardiovascular disease, disability, or dementia at baseline. Exclusion criteria include statin use in the prior year or for >5 years and inability to take a statin. Potential participants are identified using computable phenotypes derived from the electronic health record and local referrals from the community. Participants will undergo baseline cognitive testing, with physical testing and a blinded lipid panel if feasible. Cognitive testing and disability screening will be conducted annually. Multiple data sources will be queried for cardiovascular events, dementia, and disability; survival is site-reported and supplemented by a National Death Index search.
Results:
The primary outcome is survival free of new dementia or persisting disability. Co-secondary outcomes are a composite of cardiovascular death, hospitalization for unstable angina or myocardial infarction, heart failure, stroke, or coronary revascularization; and a composite of mild cognitive impairment or dementia. Ancillary studies will offer mechanistic insights into the effects of statins on key outcomes. Biorepository samples are obtained and stored for future study.
Conclusion:
These results will inform the benefit of statins for increasing survival free of dementia and disability among older adults. This is a pioneering pragmatic study testing important questions with low participant burden to align with the needs of the growing population of older adults.
Keywords: Healthy aging, older adults, dementia, statins, cognition, low-density lipoprotein-cholesterol
INTRODUCTION
Due to increasing numbers of people living into their 80s, 90s, and even 100s,1 clinical trials are needed to inform the use of interventions targeted at healthy aging. In particular, scalable ways to extend healthspan or time without dementia, disability, and cardiovascular disease (CVD) are needed.2 Conclusive evidence and guidelines support the use of statins to prevent initial and recurrent atherosclerotic CVD events in people aged <75 years. However, randomized evidence supporting the initiation of statins for primary prevention of CVD as well as cognitive impairment and disability in those ≥75 years is lacking.3–5
Statins promote cerebrovascular health and may decrease the incidence of vascular cognitive impairment and risk for Alzheimer’s disease and related dementias (ADRD).6–8 Some data suggest statins have no effect on cognitive impairment, while other data suggest statins contribute to cognitive impairment.9–12 These observational studies are limited by confounding by indication, bias in outcome ascertainment, and heterogeneity across analytic approach that leave them largely inconclusive.13,14 In addition to statins’ potential role in ADRD reduction, they may also help prevent the onset of disability. The strong association between CVD and decline in physical function suggests that statins may be useful to preserve physical function in older adults; however, previous statin trials did not enroll enough participants aged ≥75 years at risk for functional decline, thus the contribution of statins to preserve or benefit physical function in older adults is unknown.15–18 Recent evidence supports the knowledge that the risk of myalgia with statin use is infrequent, and most muscle symptoms in those on statins were similar to those on placebo.19,20 High-quality evidence for prescribing statins and other scalable measures directed at optimizing healthy life years is therefore needed.21
PREVENTABLE (PRagmatic EValuation of evENTs And Benefits of Lipid-lowering in oldEr adults) will address these knowledge gaps as the first trial to randomize older adults to a statin or placebo and follow them for a non-CVD primary outcome. This pragmatic trial is well underway to enroll 20,000 participants aged ≥75 years free of atherosclerotic CVD, dementia, or disability at enrollment. Participants are being enrolled from the Patient Centered Outcomes Research Network (PCORnet), Veterans Affairs (VA) health care system, and other participating health systems. Utilizing a double-blind, placebo-controlled randomized trial design, PREVENTABLE will evaluate the risks and benefits of a high-intensity statin compared with placebo on universally important outcomes for healthy aging. In addition, ancillary studies will offer mechanistic insights into the effects of statins on key outcomes (Supplementary Appendix). Results will guide evidence-based use of statins and other aging insights to care for this important and expanding population.
METHODS
PREVENTABLE plans to randomize 20,000 community-dwelling adults aged ≥75 years without CVD, dementia, or significant disability at baseline to receive atorvastatin 40 mg daily or matching placebo. Participants will be followed up to 6 years (estimated median 3.5–4 years). (Figure 1). Participants will be enrolled from approximately 100 large health systems and VA hospital sites. Lists generated from electronic health records (EHR) will be used to identify potentially eligible patients. We are also engaging community organizations and clinicians serving older adults to promote recruitment. Efforts to include Black/African-American and Hispanic/Latinx participants in greater numbers than in previous studies will ensure results are meaningful for these groups. The protocol was finalized in March 2020 and modified in May 2020 to allow virtual enrollment due to the COVID-19 pandemic. The first participant was enrolled on September 1, 2020.
Figure 1.
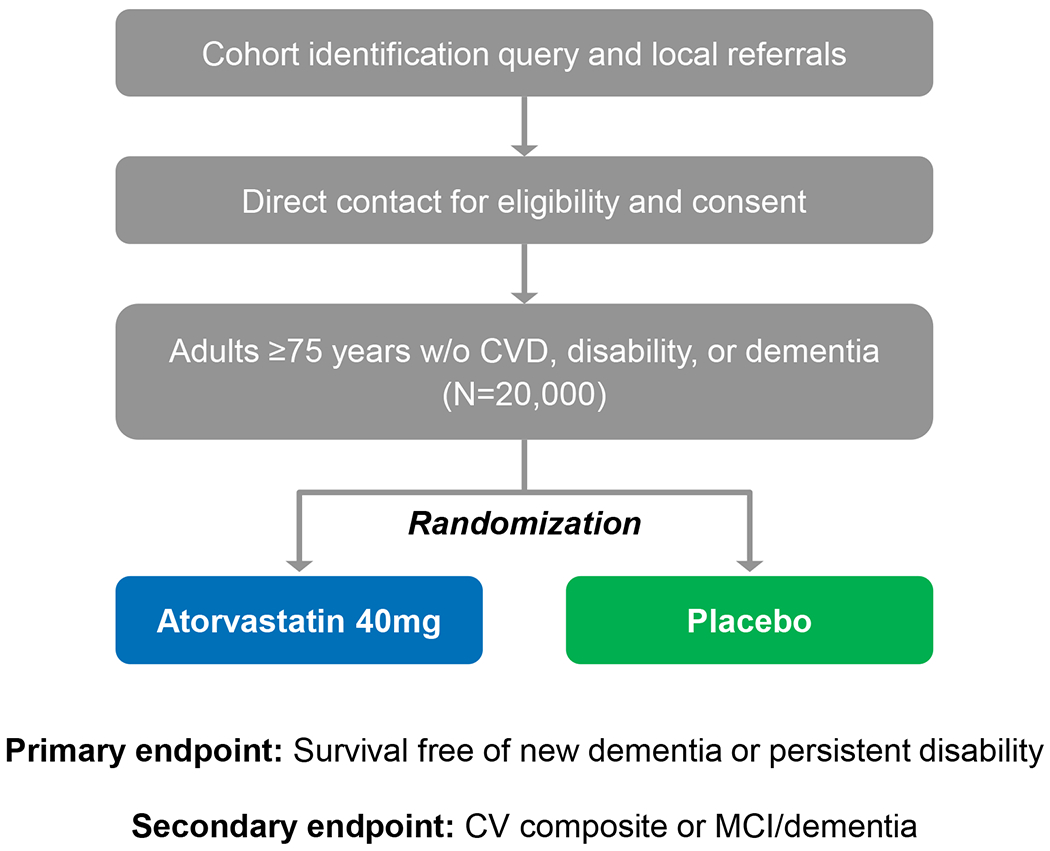
PREVENTABLE study design
PREVENTABLE aims to determine the role of a high-intensity statin in preventing dementia and prolonging disability-free survival in a broad and inclusive population of older patients; and secondarily to determine the role of high-intensity statin in preventing cardiovascular hospitalization or CVD-related death and mild cognitive impairment or dementia. The collection of biospecimens will also advance precision health in older adults. The PREVENTABLE Trial is similar to the ongoing STAREE trial in patients older than 70 years (A Clinical Trial of STAtin Therapy for Reducing Events in the Elderly in Australia; https://clinicaltrials.gov/ct2/show/NCT02099123). Despite differences in design and implementation, the similarities in the research question have the potential to enhance the understanding of outcomes overall and across subgroups.
Pragmatic Features
Trials have key features that can be placed on a continuum from explanatory to pragmatic, based on the requirements of the study. PREVENTABLE has many pragmatic features as assessed by the PRECIS-2 tool to rate domains of trial design using a scale from 1 (very explanatory) to 5 (very pragmatic).22 Trial domains included are study eligibility, recruitment, setting, organization, flexibility of delivery and adherence to intervention, follow-up, outcomes, and analysis. PREVENTABLE’s broad eligibility, flexible adherence and delivery of study drug, primary outcome, and data collection (EHR, National Death Index, and Medicare) are very pragmatic. Features that lean towards explanatory include randomization to study drug, inclusion of large institutional networks, call center collection of cognitive and disability outcomes, and the work of recruitment (Figure 2).
Figure 2.
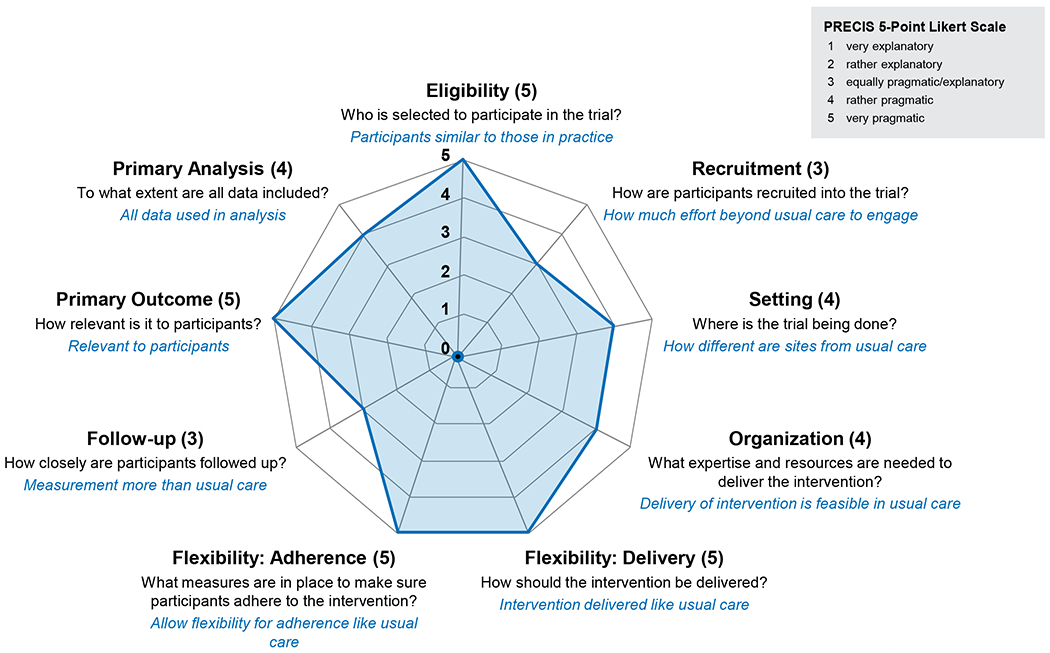
PREVENTABLE pragmatism on PRECIS-2
Patient-Stakeholders
Patient-stakeholders are included at every stage and have their own trial committee (the PREVENTERS). The patient perspective is also included in other committees to ensure the study remains patient-centered. To promote patient engagement throughout and given no in-person follow-up visits, we employ multiple avenues including newsletters, a website with patient-facing material (https://preventabletrial.org), and social media presence, in addition to the efforts of the PREVENTERS.
Study Population and Recruitment
Eligibility criteria provide for a broadly inclusive population (Table 1), with emphasis on inclusion of minority populations and older adults with multimorbidity who are traditionally under-represented in clinical trials. Financial supplements were added in recognition of the need to screen higher numbers of potentially eligible participants using multiple recruitment approaches (mail, phone, in-person) for effective recruitment. Extensive resources were allocated to support the effort required to enroll such as multiple recruitment materials in English and Spanish including brochures, flyers, postcards, Frequently Asked Questions, posters, self-mailers, greeting cards, social media presence, and weekly Zoom meetings for interested participants.
Table 1.
PREVENTABLE inclusion and exclusion criteria
| Inclusion Criteria | • Community-dwelling adults • Age ≥75 years • English or Spanish as primary language |
| Exclusion Criteria | • Clinically evident cardiovascular disease defined as prior myocardial Infarction, prior stroke, prior revascularization procedure, or a secondary prevention indication for a statin (clinician determined) • Hospitalization for a primary diagnosis of heart failure in the prior 12 months (Note: History of heart failure in the absence of recent hospitalization or clinically evident cardiovascular disease is not an exclusion) • Dementia (clinically evident or previously diagnosed) • Dependence in any Katz Basic Activities of Daily Living (with the exception of urinary or bowel continence) • Severe hearing impairment (preventing phone follow up) • Unable to talk (preventing phone follow up) • Statin use in the past year or for longer than 5 years previously (participant reported) • Ineligible to take atorvastatin 40 mg (clinician determined) • Documented intolerance to statins • Active liver disease |
Study Treatment
Participants are centrally randomized via a random number generator 1:1 to atorvastatin 40 mg or matching placebo taken once daily. Atorvastatin 40 mg was chosen based on evidence of efficacy and common use. In pivotal clinical trials for regulatory approval, 39,828 patients received atorvastatin doses ranging from 10–80 mg, of which 2800 patients (7%) were ≥75 years (Supplementary Table 1).23 In these studies, there were no differences in safety or effectiveness of atorvastatin in younger versus older patients. Atorvastatin 40 mg daily is a high-intensity statin dose that leads to a 50% low density lipoprotein cholesterol (LDL-C) reduction, is well tolerated, and will avoid the risk of under-treatment as a potential concern.24,25 In practice, older adults tolerate statins similarly to younger adults supporting tolerability of this dose selection.26 PREVENTABLE was determined to be exempt from the Investigational New Drug (IND) regulations [21 CFR 312.2 (b)(1)].
The VA Cooperative Studies Program Clinical Research Pharmacy Coordinating Center (Albuquerque, NM) is the study central pharmacy. The study central pharmacy acquires atorvastatin 40 mg tablets, manufactures matching placebo tablets, and packages products into bottles. Following receipt of an order for study drug, study drug is labeled and shipped directly to participants every 90 days via the United States Postal Service (USPS). Orders for study drug are renewed either annually (non-VA sites) or every 3 months (VA sites), at which time the site investigator confirms continuing eligibility for study drug. Data integrations between the study database, the VA, the study central pharmacy, and USPS facilitate order transmission as well as communication about shipping address changes, discontinuation of study drug, and shipment delivery. Participants, treating clinicians, study team, and personnel involved in endpoint capture are unaware of treatment allocation group.
Schedule of Events
PREVENTABLE streamlines work for the participant and study site (Table 2). The site uses a computable phenotype, developed specifically for the study by the Data Coordinating Center and the VA Network Coordinating Center, to identify and generate recruitment lists of potentially eligible participants. The computable phenotype includes code lists and logic that sites use to implement the study eligibility criteria while querying their EHR data. Using in-person or remote contact, the site confirms eligibility and enrolls those who consent. The site is also responsible for annual confirmation of suitability to continue receiving study drug. Data queries on hospitalizations and laboratory testing will be used for safety and endpoint determination. The Geriatrics Outcomes Assessment Center at Wake Forest University School of Medicine (Winston-Salem, NC) is responsible for baseline and annual phone-based assessments of cognitive and physical function. During the enrollment visit, a member of the study team reviews study information and collects baseline medical history. If the visit is in-person, they also collect blood and the Short Physical Performance Battery (SPPB). Trained personnel at Wake Forest collect the baseline memory tests. A subset of study participants (approximately 2,000) will return for a repeat lipid panel at three months. If the participant enrolls virtually, the lipid panel and physical assessments will not be completed. After randomization, all follow-up procedures such as harvesting data from the medical record and phone calls and screening/evaluation for dementia and disability are performed centrally. The study drug is mailed directly to the participant reducing participant burden. Participants are provided a handout and a wallet card that explain what they should do and who to contact if they experience any new symptoms or side effects while participating in the trial. The primary care clinician is made aware of their patient’s study participation and are included in treatment decisions as needed.
Table 2.
Schedule of PREVENTABLE study visits
| Baseline (Visit 1) |
Baseline Call (Visit 2) |
Follow-Up (Visit 3) |
Follow-Up Call (Visit 4) |
Follow-Up Call (Visit 5) |
Follow-Up Call (Visit 6) |
Final Visit Call (Visit 7) |
|
|---|---|---|---|---|---|---|---|
| Timeline | 0 | 2 wks | 3 mo | 12 mo | 24 mo | 36 mo* | EOS |
| Informed Consent | X | ||||||
| Study Enrollment | X | ||||||
| Randomization | X | ||||||
| Demographics, Medical History | X | ||||||
| ADL Screen | X | ||||||
| Study Blood Draw - Lipid Panel | X† | X‡ | |||||
| Study Blood Draw – Biorepository | X† | ||||||
| Use/Eligibility of Study Drug (Site) | X | X | X | X | |||
| Review of Eligibility for Study Drug | X | X | X | ||||
| Cognitive Function | X | X | X | X | X | ||
| Physical Function (ADL and PROMIS-PF) | X | X | X | X | X | ||
| SPPB | X† | ||||||
Then every 12 months until EOS.
For participants enrolled by telehealth, blood draw and SBBP may be obtained separately in-person.
Subset of participants.
ADL indicates activities of daily living; EOS, end of study; SPPB, short physical performance battery.
Safety
Patient-level meta-analysis from randomized trials and placebo-controlled crossover studies in statin-intolerant patients document the attribution of side effects to placebo further supporting the tolerability of statins.19,20,27 When safety concerns arise, they will be addressed by the study team in collaboration with routine healthcare follow-up and treatment. Data is collected through the EHR, rather than site reporting of adverse events. Reporting will be governed by the Common Rule (45 CFR Part 46, Subpart A), as well as International Council for Harmonization Guidelines, institutional review boards (IRBs), and local regulations. In addition, an independent data safety monitoring board (DSMB) appointed by the National Institute on Aging (NIA) reviews aggregate safety events, primary and secondary endpoints, reasons for stopping study drug, hospitalizations, events of special interest, and deaths. In addition to vital status, events of special interest such as new-onset diabetes, hepatic failure, myositis, and cancer are reported to the DSMB. The DSMB reviews rates in each treatment group to inform their recommendation to continue or stop the study or modify the protocol. Circumstances that warrant termination or suspension include, but are not limited to, unexpected, significant, or unacceptable risk to participants, inadequate compliance with protocol requirements, incomplete or unevaluable data, or determination of futility by the DSMB.
Biorepository
The Biorepository Core provides coordination and logistical support for collection, processing, and storage of baseline samples from randomized participants and samples from 2000 participants in follow-up. Supplementary Figure 1 outlines the baseline specimen collection. Each participant able to participate in the biorepository collection provides a total of 20 cc whole blood that will include a 10 cc ethylenediaminetetraacetic acid tube (for plasma and buffy coat) and a 10 cc red top (for serum) for a total of 9 aliquots available for future studies. Participation in the biorepository sample collection was optional for participants enrolled virtually due to the pandemic.
Trial Organization
The study is overseen by the Steering Committee, along with its subcommittees, in partnership with the NIA and National Heart, Lung, and Blood Institute (NHLBI). The Steering Committee includes representatives from clinical sites and core operational groups (Biorepository; Recruitment, Retention, and Adherence; Geriatric Outcomes Assessment; Ancillary Studies; Central Pharmacy; and the PREVENTERS). The Clinical Coordinating Center at Duke University (Durham, NC) is responsible for study coordination, site management, communication, single IRB coordination, and financial administration. The Data Coordinating Center is responsible for the treatment allocations, electronic case report forms, study website, receipt and processing of data, quality control programs, coordination and tracking for central units, and statistical analysis and reporting. Committee members are listed in the Supplementary Appendix. In accordance with the NIH’s single IRB mandate for multicenter research, the single IRB of record for non-VA sites is the Duke University IRB (Durham, NC) and for the VA sites is the Veterans Affairs Central IRB (cIRB) (Washington, DC).
Outcomes Definitions
Primary and Secondary Outcomes
The primary outcome is survival free of new dementia or persistent disability, ascertained as noted below. The co-secondary outcomes are (1) the composite of cardiovascular death, hospitalization for unstable angina or myocardial infarction, heart failure, stroke, or coronary revascularization; and (2) the composite of mild cognitive impairment (MCI) or dementia.
Cognitive Outcomes
Participants will be categorized at baseline and follow-up as having no cognitive impairment (NCI), MCI, or probable dementia. A phone cognitive battery will be administered by the Central Call Center to all participants using the Telephone Interview for Cognitive Status-Modified (TICS-M)28,29 which will be repeated annually during follow-up. Participants suspected of having possible cognitive impairment will undergo The Extended Cognitive Assessment Battery (National Alzheimer’s Coordinating Center Uniform Data Set Version 3).30 For more detailed assessment of cognitive functions along with the Patient Health Questionnaire (PHQ)-8,31 the Functional Assessment Questionnaire (FAQ) will be administered to a trusted contact familiar with the participant’s daily function.32 All tests, questionnaires, and data from the EHR and Medicare claims relevant to cognitive impairment will be submitted to a centralized, web-based system for adjudication by a panel of dementia experts who will assign final study classifications of NCI, MCI, or probable dementia.33
At annual follow-up, cognitive assessment will generally use the same process as baseline, with the exception of adding the Dementia Questionnaire (DQ) administered to a previously identified trusted contact if the participant passes away or otherwise cannot be contacted.32,34 The DQ will be administered if it has been more than 6 months since the participant’s last planned cognitive assessment. Annual cognitive assessments will stop after a participant is classified as having probable dementia. Details about the criteria to be used for classifying incident MCI and probable dementia are available in the Supplementary Appendix. Even though an adjudicated event is not equivalent to a clinical diagnosis, the site study team will be notified of probable dementia adjudication; if the participant has given permission, the study team will notify the primary clinician for clinical evaluation.
Functional Outcomes
Persistent disability is defined as loss of independence in one or more basic activities of daily living (ADL), except for urinary or bowel continence, at 2 visits at least 3 months apart (to exclude transient loss of function), reported by the participant or trusted contact.35,36 Since muscle-related limitation due to statin use is a concern in this trial, we administer the Short Performance Physical Battery (SPPB) at baseline that includes assessment of lower extremity function if possible.37 Functional assessment will also include telephone screening for physical function and disability using the PROMIS 20-item physical functioning scale at baseline and annually thereafter or until a participant is classified as having a persistent disability.38 A decline in PROMIS physical functioning scale of ≥2 points is associated with subsequent disability. Similar to cognitive outcomes, the Katz ADL will be administered to a trusted contact and used to assess disability if other methods are not possible. Any report of new dependence ≥1 Katz ADL will be confirmed 3 months later in order to classify as new persistent disability.
All-cause Mortality
Mortality data will be captured from the site death report form, Medicare beneficiary status change, and National Death Index (NDI). If the central study teams are the first to learn of a participant’s death, that information will be relayed to the site.
Cardiovascular Outcomes
Deaths will be captured and classified as cardiovascular or non-cardiovascular (malignancy or “other”) using site death report form and NDI data, complemented by hospitalization records as necessary and available. Cardiovascular hospitalizations (myocardial infarction, unstable angina, coronary revascularization, heart failure, and stroke) will be captured from EHR databases complemented by Medicare claims data.
Other Outcomes
We will capture all-cause hospitalizations and days spent at home.39,40 Individual components of the primary outcomes such as self-reported physical function derived from PROMIS-PF and cognitive function based on TICS-M will also be evaluated as independent secondary endpoints.
Statistical Analysis
Primary Outcome
Based on the intention-to-treat principle, we will compare treatment groups using a Cox proportional hazards regression model, stratifying the baseline hazard function by site.41 The hazard ratio from this model, with associated 95% confidence intervals, will be our primary measure of treatment effect. We will not formally test the proportional hazards assumption of the Cox model,42 instead we will compute complementary treatment group estimates using the restricted mean survival time, calculated at 2 and 4 years of follow-up.43
Secondary Outcomes
Given the competing risk of non-cardiovascular death, analyses for the cardiovascular secondary outcome will be based on the subdistribution hazard model of Fine-Gray,44 stratified by clinic site. We will follow recommendations for the reporting of such analyses,45 describing hazard ratios from the Fine-Gray model with respect to the cumulative incidence function for the event of interest. The secondary outcome of MCI or probable dementia is subject to both interval-censoring due to intermittent ascertainment, as well as the competing risk of death. We will therefore utilize the same framework of the Fine-Gray subdistribution hazard model, combined with sensitivity analyses using multiple imputation to address the influence of interval-censoring.46,47 Hypothesis tests for secondary endpoints will be 2-sided, employing an unequal allocation of the alpha level to control the type I error rate for the secondary hypotheses. Because we expect a higher event rate for the composite of MCI or probable dementia versus CVD, the alpha level for the secondary endpoints will be partitioned unevenly as 4% for CVD and 1% for MCI/probable dementia.
LDL-C Reductions
Adherence will be determined in a pragmatic manner utilizing the number of days a participant had medication available during the study period. We will also estimate the magnitude of achieved reductions in LDL-C with atorvastatin 40 mg by comparing changes in LDL-C levels between baseline and 3 months of follow-up in the lipid panel subgroup (n=2000). These analyses will be based on linear mixed models using 3-month LDL-C as the outcome, incorporating site-specific random effects, and adjusting for baseline LDL-C levels. We will report absolute and percent reductions in LDL-C.
Subgroups
Recognizing that analyses of treatment effect heterogeneity are typically underpowered, a limited number of pre-specified subgroup analyses will be conducted for the primary and secondary outcomes. Analyses will include formal tests of interaction within Cox regression or Fine-Gray subdistribution hazard models as appropriate. The nominal p-value for the interaction term using a likelihood ratio test will be reported along with within subgroup estimates of the intervention effect and associated nominal 95% confidence intervals. Subgroups of interest, defined according to baseline characteristics, will include: sex; race; ethnicity; estimated life expectancy based on the modified Lee Index48 (≤7 [<20% risk of 5-year mortality], 8–12 [20 to ≤50% 5-year mortality risk], or >12 [≥50% 5-year mortality risk]; baseline physical function (if available) based on the Short Physical Performance Battery (<10 versus ≥10)37; multimorbidity (median split); baseline LDL-C (median split); and diabetes at baseline. Finally, to control for multiplicity amongst the pre-specified subgroups, we will also report adjusted p-values based on the Holm sequential procedure.49
Sample Size and Statistical Power
Primary Outcome
Power calculations were informed by data in adults ≥75 years without a history of CVD from the Systolic Blood Pressure Intervention Trial (SPRINT)50 and the Aspirin in Reducing Events in the Elderly (ASPREE) trial.51 These trials indicated an expected CVD rate of roughly 30–40 events per 1000 person-years. We further assumed that atorvastatin 40 mg would lead to a 20% reduction in the primary outcome in a hypothetical scenario with full adherence. However, non-negligible cross-over between the treatment groups is certainly expected, driven by statin intolerance and placebo participants experiencing CVD events that would warrant a statin for secondary prevention. Medication data from Australia indicated that in adults ≥75 years, approximately 27.3% of those initiating a statin will discontinue use of the medication over a mean follow-up of 5 years.52 However, amongst those who discontinue, about 25% will reinitiate statin treatment. Based on these estimates, we assumed that 20% of participants randomized to atorvastatin would discontinue and not reinitiate statin treatment. Similarly, we assumed that 10% of participants randomized to placebo would initiate statin treatment during follow-up. These assumptions imply that cross-over would reduce the assumed treatment effect of a 20% reduction to 14.3% (hazard ratio=0.857).53 Assuming a total study length of 5 years, a 2-year recruitment period, and 3% loss to follow-up per year, we estimated that 20,000 participants would provide 94.2% power (assuming an event rate of 32.6 per 1000 person-years based on ASPREE). These estimates were subsequently updated to reflect a longer anticipated recruitment window (~54 months [4.5 years]), with a total study length of 6.5 years, which increases the power estimate to 95.1%. We have considered sensitivity estimates examining higher rates of statin discontinuation. If the discontinuation rate in those randomized to atorvastatin increases to 25%, this reduces the assumed treatment effect to a hazard ratio of 0.867, and decreases power to 91.7%. Similarly, increasing the discontinuation rate to 30% decreases the assumed hazard ratio to 0.877, and reduces power to 86.9%.
Secondary Outcomes
Power for the composite secondary outcome of MCI or probable dementia used analogous calculations, with two primary changes to the assumptions. First, the inclusion of MCI implies a higher expected event rate, estimated to be 61.2 events per 1000 person-years based on SPRINT. Second, this event rate was approximately double what we expected to observe for the secondary cardiovascular endpoint. Therefore, we planned an unequal partition of the type I error rate, allocating 1% to the MCI/probable dementia composite and 4% to the CVD endpoint. Based on these assumptions, we estimated that 20,000 participants would have 98.3% power to detect a 14.3% reduction in the combined incidence of MCI or probable dementia.
With respect to CVD, meta-analyses indicate an approximate 22% reduction in the risk of major atherosclerotic CVD events per 1 mmol/L (39 mg/dL) reduction in LDL-C.24,54 We estimated that the baseline LDL-C levels in the cohort would be 110–120 mg/dL (2.84–3.10 mmol/L). Assuming a 50% LDL-C reduction with atorvastatin 40 mg (i.e., mean decreases of 1.42–1.55 mmol/L), this would correspond to about a 30% relative risk reduction (hazard ratio=0.70). With similar assumptions for cross-over as for primary outcome (20% statin discontinuation and 10% cross-over from placebo), this reduces the assumed effect to a hazard ratio of 0.783. There is some uncertainty about the expected incidence of CVD in this population, as estimates vary widely from trials like ASPREE (14.1 events per 100 person-years) versus SPRINT (31.2 events per 100 person-years).33 A sample size of 20,000 participants would provide >99.0% power. Using the rate from SPRINT, though this is reduced to 95.6% assuming the lower assumed rate from ASPREE. If the strength of the assumed treatment effect is reduced to a hazard ratio of 0.857 (consistent with the primary outcome), then power is reduced to 95.01% (SPRINT rate) or 64.7% (ASPREE) rate.
Changes as a result of COVID-19
The trial adapted to the significant challenges to in-person recruitment due to COVID-19 by rapidly facilitating and encouraging the adoption of remote consenting. The requirement for baseline labs and SPPB was waived, and additional payment was provided to sites that could obtain labs and SPPB. In addition to e-consent platform for enrolling participants at non-VA sites, new methods were added for remote consenting at all sites. Novel approaches included phone consenting and the use of DocuSign for remote consenting, the use of VA Video Connect for virtual study visits, and Rights Management System (RMS) Outlook encryption to enable electronic communication with participants and family members. To mitigate the effects of study staff turnover at sites, the VA National Network provided support for regulatory activities. Although not included in the original site budgets, an allowance for postage was added later to facilitate the remote outreach that was needed to contact older adults who were often unwilling to attend in-person screening visits.
Conclusion
PREVENTABLE is a landmark initiative to address a question of vital importance to old and very old US adults with multiple stakeholders including the participants themselves. This study will inform the benefit of initiating a high-intensity statin for the primary prevention of death, dementia, and physical disability as well as MCI and CVD in adults older than 75 years, especially in those with concomitant multi-morbidity and frailty. With a biorepository and ancillary studies, there will be important opportunities for knowledge generation for dementia and cardiovascular science in older adults to inform which subgroups may benefit the most. The trial also brings the rigor of traditional randomized controlled trials, with randomization, a double-blind and placebo-controlled intervention, and centralized primary outcome ascertainment as well as more pragmatic elements with real-world data for outcomes and pragmatic study drug adherence. The primary study question seeks to identify a foundational and scalable way to increase quality independent life years among older adults without dementia or cardiovascular disease at baseline.
Supplementary Material
Key Points:
Clinical trials tailored to questions of importance to healthy older adults are urgently needed due to increasing numbers of people living into their 80s, 90s, and even 100s.
Whether statins could prolong healthy life years without dementia and disability in adults aged ≥75 years is unknown.
PREVENTABLE is the largest trial conducted in adults ≥75 years in the United States and is tailored to answer a key clinical question while limiting participant burden
Why does this matter?
This pragmatic trial is employing methodologies that limit burden on participants while also obtaining high quality evidence in support of the effectiveness of the intervention on key study outcomes. Results will establish whether initiating a high-intensity statin is effective in the older population without heart disease or dementia to improve healthspan.
ACKNOWLEDGMENTS
The Alzheimer’s Association contributes to study leadership, supports investigator meetings, and facilitates awareness through local and national channels.
Funding:
Preventable is supported by a U19 AG065188 from the National Institute on Aging (NIA) with contribution from the National Heart, Lung, and Blood Institute (NHLBI). This study was conducted using PCORnet®, the National Patient-Centered Clinical Research Network. PCORnet® has been developed with funding from the Patient-Centered Outcomes Research Institute® (PCORI®). This project was also supported in part by the Duke University-Vanderbilt University Medical Center Trial Innovation Center (U24TR001608 -NCATS, Trial Innovation Network). This work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Sponsor’s Role:
The National Institute on Aging program staff had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript. They did not have a role in the decision to submit the manuscript for publication. This material is the result of work supported with resources and the use of facilities of the Department of Veterans Affairs. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
Conflict of Interest:
Thomas M. Gill is supported by P30AG021342 and 5UL1TR001863. Dr. Pajewski is supported by P30AG021332, R01HL155396, and R01AG071807. Dr Roumie is supported in part by CX000570-10 and the Center for Diabetes Translation Research P30DK092986.
Daniel E. Forman is supported by NIH awards R01AG060499, R01AG058883, and P30AG02482, and VHA MERIT award I01HX003518.
L. Kristin Newby reports research funding from NHLBI, NIA, NICHD, NC DHHS, BioKier, Roche Diagnostics; Consulting/advisory board for CSL-Behring, Medtronic, Beckman-Coulter, NHLBI; Non-funded “other” for BOD DHMRI, BOT AZ Healthcare Foundation.
Jacob Joseph reports research funding from NIH, Veterans Health Administration, Amgen, Kowa, and Alnylam.
Kenneth Schmader reports support from the Duke Pepper Older Americans Independence Center (NIA P30AG028716).
Michael Shapiro reports scientific advisory boards: Amgen, Novartis, NovoNordisk; Consulting: Ionis, Regeneron.
W. Schuyler Jones reports research grants from Bayer Boehringer Ingelheim, Merck, National Institutes of Health, Patient-Centered Outcomes Research Institute; Honoraria/Advisory Committees from Bayer, Bristol-Myers Squibb, Janssen Pharmaceuticals.
Catherine P. Benzinger reports site PI for studies funded by Department of Defense (WARRIOR), Amgen (VESALIUS), Novartis (INCLISIRAN), but are not relevant to the current study (does not receive financial support).
Raj C. Shah reports being the site principal investigator or sub-investigator for Alzheimer’s disease clinical trials for which his institution (Rush University Medical Center) is compensated (Amylyx Pharmaceuticals, Inc., Athira Pharma, Inc., Edgewater NEXT, Eli Lilly & Co., Inc., and Genentech, Inc.).
Christine E. Kistler reports serving as a consultant to a pharmacogenomics company, Base10, Inc. and on a pharmacogenomics advisory board for Baptist Health, FL.
Ariela R. Orkaby reports Veterans Affairs CSR&D CDA-2 award IK2-CX001800; consulting for Anthos Therapeutics.
Rowena J. Dolor reports grant funding from NIA paid to the institution for this project; PCORI, honorarium (less than $5000), as a member of the PCORI Clinical Trials Advisory Panel; Einstein-Montefiore, honorarium (less than $5000), as a member of their CTSA External Advisory Board; Veterans Administration, honorarium (less than $5000), as a consultant to the Women’s Health Practice-Based Research Network.
Karen P. Alexander reports grant funding from NIA to the institution for this project.
All other authors have nothing to report.
Footnotes
Trial Registration: ClinicalTrials.gov Identifier: NCT04262206 (https://clinicaltrials.gov/ct2/show/NCT04262206)
REFERENCES
- 1.Census.gov. Available at: https://www.census.gov/content/dam/Census/library/publications/2020/demo/p23-217.pdf. Accessed October 18, 2022.
- 2.Bernard MA, Clayton JA, Lauer MS. Inclusion Across the Lifespan: NIH Policy for Clinical Research. JAMA 2018;320:1535–1536. [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Zieman S, Go AS, et al. Statins for Primary Prevention in Older Adults-Moving Toward Evidence-Based Decision-Making. J Am Geriatr Soc 2018;66:2188–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangione CM, Barry MJ, Nicholson WK, et al. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: US Preventative Services Task Force Recommendation Statement. JAMA 2022;328:746–753. [DOI] [PubMed] [Google Scholar]
- 5.Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015;162:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mok VCT, Lam WWM, Fan YH, et al. Effects of statins on the progression of cerebral white matter lesion: Post hoc analysis of the ROCAS (Regression of Cerebral Artery Stenosis) study. J Neurol 2009;256:750–757. [DOI] [PubMed] [Google Scholar]
- 7.Bath PM, Scutt P, Blackburn DJ, et al. Intensive versus Guideline Blood Pressure and Lipid Lowering in Patients with Previous Stroke: Main Results from the Pilot ‘Prevention of Decline in Cognition after Stroke Trial’ (PODCAST) Randomised Controlled Trial. PLoS One 2017;12:e0164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bettermann K, Arnold AM, Williamson J. et al. Statins, risk of dementia, and cognitive function: secondary analysis of the ginkgo evaluation of memory study. J Stroke Cerebrovasc Dis 2012;21:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ott BR, Daiello LA, Dahabreh IJ, Springate BA, et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med 2015;30:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumurgier J, Singh-Manoux A, Tavernier B, Tzourio C, Elbaz A. Lipid-lowering drugs associated with slower motor decline in the elderly adults. J Gerontol A Biol Sci Med Sci 2014;69:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurwitz JH, Go AS, Fortmann SP. Statins for Primary Prevention in Older Adults: Uncertainty and the Need for More Evidence. JAMA 2016;316:1971–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Z, Ryan J, Ernst ME, et al. Effect of statin therapy on cognitive decline and incident dementia in older adults. J Am Coll Cardiol 2021;77:3145–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adhikari A, Tripathy S, Chuzi S, Peterson J, Stone NJ. Association between statin use and cognitive function: a systematic review of randomized clinical trials and observational studies. J Clin Lipidol 2021;15:22–32. [DOI] [PubMed] [Google Scholar]
- 14.Power MC, Weuve J, Sharrett AR, Blacker D, Gottesman RF. Statins, cognition, and dementia - systematic review and methodological commentary. Nat Rev Neurol 2015;11:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Hazzouri AZ, Jawadekar N, Grasset L, et al. Statins and Cognitive Decline in the Cardiovascular Health Study: A Comparison of Different Analytic Approaches. J Gerontol A Biol Sci Med Sci 2022;77:994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd-Jones DM, Braun LT, Ndumele CE, et al. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Special Report From the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2019;73:3153–3167. [DOI] [PubMed] [Google Scholar]
- 18.Greenland P, Michos ED, Redmond N, et al. Primary Prevention Trial Designs Using Coronary Imaging: A National Heart, Lung, and Blood Institute Workshop. JACC Cardiovasc Imaging 2021;14:1454–1465. [DOI] [PubMed] [Google Scholar]
- 19.Cholesterol Treatment Trialists’ Collaboration. Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large scale randomized double-blind trials. Lancet 2022;400:832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard JP, Wood FA, Finegold JA, et al. Side effect patterns in a crossover trial of statin, placebo, and no treatment. J Am Coll Cardiol 2021;78:1210–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weir K, Nickel B, Naganathan V, et al. Decision-Making Preferences and Deprescribing: Perspectives of Older Adults and Companions About their Medicines. J Gerontol B Psychol Sci Soc Sci 2018;73:e98–e107 [DOI] [PubMed] [Google Scholar]
- 22.Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350:h2147. [DOI] [PubMed] [Google Scholar]
- 23.US FDA. Atorvastatin Package Insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020702s056lbl.pdf. Accessed October 11, 2022.
- 24.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridker PM, Lonn E, Paynter NP, Glynn R, Yusuf S. Primary Prevention With Statin Therapy in the Elderly: New Meta-Analyses From the Contemporary JUPITER and HOPE-3 Randomized Trials. Circulation 2017;135:1979–1981. [DOI] [PubMed] [Google Scholar]
- 26.Nanna MG, Navar AM, Wang TY, et al. Statin Use and Adverse Effects Among Adults >75 Years of Age: Insights From the Patient and Provider Assessment of Lipid Management (PALM) Registry. J Am Heart Assoc 2018;7:e008546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrett E, Williamson E, Brack K, et al. Statin treatment and muscle symptoms: series of randomised, placebo controlled n-of-1 trials. BMJ. 2021;372:n135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandt JM, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiat Neuropsychol Behav Neurol 1988;1:111–117. [Google Scholar]
- 29.Welsh KA, Breitner JC, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiat Neuropsychol Behav Neurol 1993;6:103–110. [Google Scholar]
- 30.Besser L, Kukull W, Knopman DS, et al. Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis Assoc Disord 2018;32:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–173. [DOI] [PubMed] [Google Scholar]
- 32.Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A validation study of the Dementia Questionnaire. Arch Neurol 1994;51:901–906. [DOI] [PubMed] [Google Scholar]
- 33.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. JAMA 2016;315:2673–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellis RJ, Jan K, Kawas C, et al. Diagnostic validity of the dementia questionnaire for Alzheimer disease. Arch Neurol 1998;55:360–365. [DOI] [PubMed] [Google Scholar]
- 35.Gill TM. Assessment of function and disability in longitudinal studies. J Am Geriatr Soc 2010;58 Suppl 2:S308–S312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970;10:20–30. [DOI] [PubMed] [Google Scholar]
- 37.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 38.Hays RD, Spritzer KL, Fries JF, Krishnan E. Responsiveness and minimally important difference for the patient-reported outcomes measurement information system (PROMIS) 20-item physical functioning short form in a prospective observational study of rheumatoid arthritis. Ann Rheum Dis 2015;74:104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groff AC, Colla CH, Lee TH. Days Spent at Home - A Patient-Centered Goal and Outcome. N Engl J Med 2016;375:1610–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee H, Shi SM, Kim DH. Home Time as a Patient-Centered Outcome in Administrative Claims Data. J Am Geriatr Soc 2019;67:347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glidden DV, Vittinghoff E. Vittinghoff, Modelling clustered survival data from multicentre clinical trials. Stat Med 2004;23:369–388. [DOI] [PubMed] [Google Scholar]
- 42.Stensrud MJ, Hernan MA. Why Test for Proportional Hazards? JAMA 2020;323:1401–1402. [DOI] [PubMed] [Google Scholar]
- 43.Zhao L, Claggett B, Tian L, et al. On the restricted mean survival time curve in survival analysis. Biometrics 2016;72:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 45.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017;36:4391–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu B, Saczynski JB, Launer L. Multiple imputation for estimating the risk of developing dementia and its impact on survival. Biom J 2010;52:616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curnow E, Hughes RA, Birnie K, et al. Multiple imputation strategies for a bounded outcome variable in a competing risks analysis. Stat Med 2021;40:1917–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kotwal AA, Lee SJ, Dale W, Boscardin WJ, Waite LJ, Smith AK. Integration of an Objective Cognitive Assessment Into a Prognostic Index for 5-Year Mortality Prediction. J Am Geriatr Soc 2020;68:1796–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holm S A Simple Sequentially Rejective Multiple Test Procedure. Scand J Stat 1979;6:65–70. [Google Scholar]
- 50.Williamson JD, Pajewski NM, Auchus AP, et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA 2019;321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNeil JJ, Woods RL, Nelson MR, et al. Effect of Aspirin on Disability-free Survival in the Healthy Elderly. N Engl J Med 2018;379:1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ofori-Asenso R, Ilomaki J, Tacey M, et al. Switching, Discontinuation, and Reinitiation of Statins Among Older Adults. J Am Coll Cardiol 2018;72:2675–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wittes J Sample size calculations for randomized controlled trials. Epidemiol Rev 2002;24:39–53. [DOI] [PubMed] [Google Scholar]
- 54.Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019;393:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


