Abstract
Pomegranate (Punica granatum L.) fruits are a historical agricultural product of the Mediterranean basin that became increasingly popular in the latest years for being rich in antioxidants and other micronutrients, and are extensively commercialized as fruits, juice, jams and, in some Eastern countries, as a fermented alcoholic beverage. In this work, four different pomegranate wines specifically designed using combinations of two cultivars (Jolly Red and Smith) and two yeast starters with markedly different characteristics (Saccharomyces cerevisiae Clos and Saccharomyces cerevisiae ex-bayanus EC1118) were analyzed. The chemical characterization of the wines together with the originating unfermented juices was performed by 1H NMR spectroscopy metabolomic analysis. The full spectra were used for unsupervised and supervised statistical multivariate analysis (MVA), namely Principal Component Analysis (PCA), Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA), and sparse PCA (SPCA). The MVA of the wines showed a clear discrimination between the cultivars, and a smaller, yet significant, discrimination between the yeasts used. In particular, a higher content of citrate and gallate was observed for the Smith cv. and, on the contrary, a statistically significant higher content of fructose, malate, glycerol, 2,3 butanediol, trigonelline, aromatic amino acids and 4-hydrophenylacetate was observed in Jolly Red pomegranate wines samples. Significant interaction among the pomegranate cultivar and the fermenting yeast was also observed. Sensorial analysis was performed by a panel of testing experts. MVA of tasting data showed that the cultivar significantly affected the organoleptic parameters considered, while the yeast had a minor impact. Correlation analysis between NMR-detected metabolites and organoleptic descriptors identified several potential sensorially-active molecules as those significantly impacting the characteristics of the pomegranate wines.
Keywords: 1H NMR spectroscopy, Multivariate statistical analysis, Saccharomyces cerevisiae, Punica granatum L., Pomegranate wine, PCA, OPLS-DA, Sparse PCA
Graphical abstract

1. Introduction
In these times of health awareness, healthy eating, and nutraceutical products, pomegranate fruits experienced a great growth of commercial popularity. Pomegranate (Punica granatum L.) is a plant belonging to the family Punicaceae native of Western and Central Asia, from Iran to northern India. It has a lengthy history of cultivation throughout the whole Middle East, Indian subcontinent, and Mediterranean regions of Africa, Asia, and Europe [1]. Both the tree and the fruit of pomegranate had a wide range of uses in ancient therapeutics, together with a symbolic role in art, literature, and religion of the cultures, representing fundamental dualities, with the medicinal purposes for which pomegranate was reflecting symbolic associations as well as its pharmaceutical properties [2]. Pomegranates are thought to have been domesticated in the Transcaucasia-Caspian region, namely in the northeastern Turkey and the south Caspian regions [3]. Chemical evidences of consumption of pomegranates and possibly wine production were found in the Areni-1 cave complex in southeastern Armenia, dated around 4000 BCE (Late Chalcolithic), presumably the first evidence of plant domestication [4]. Archaeological evidence such as carbonized pips and fragments of pomegranate peels have been found from early Bronze Age Jericho and Arad, and remains of Punica species have been found in Nimrud [3]. Pomegranates were widely used in Egypt, as documented by occurrences in texts, artistic depictions, and dried fruits in tombs, Israel, Cyprus, Crete, and mainland Greece [5]. The crop was naturalized in the Mediterranean region from Phoenicians around 2000 BCE [6]. Plinius the Elder reported in his Naturalis Historiae that Carthage supplied Rome with large seedless pomegranates, called malum punicum or granatum [7]. Nowadays, pomegranate is widely cultivated in Mediterranean, tropical and subtropical regions, and in central Asia.
In recent years, the global demand for pomegranate products is greatly increased, thanks to their well-studied nutritional and organoleptic properties [8,9]. In fact, the fruit is a rich source of bioactive compounds such as phenolics, hydrolysable tannins, anthocyanins, flavonoids, and essential micro-nutrients such as vitamin C. The chemical composition is strictly related to cultivar, growing region, climate, maturity, cultivation practice, and storage conditions [1]. Among flavonoids, anthocyanin pigments are the largest and most important group present in pomegranate arils, which are used to obtain the juice. These molecules are responsible of the red colour of fruit and juice. In the group of phenolic acids, hydroxybenzoic (gallic and ellagic acids) and hydroxycinnamic acids (caffeic acid, chlorogenic acid, and p‐coumaric acid) represent the main groups [1]. A relevant content of hydrolysable tannins mainly punicalagin, pedunculagin, and punicalagin could be found in pomegranate peel [10]. The presence of significant amounts of bioactive compounds, assures this fruit exhibits strong antioxidative, anti-inflammatory, apoptotic, and antimutagenic properties [11]. The regular consumption of this fruit is associated with prevention of gastric damage, cardiovascular disease, type 2 diabetes, specific types of cancers, renal illnesses, liver complications, and osteoarthritis [11].
Edible parts of pomegranate fruits traditionally are eaten by removing them manually after breaking the fruit hard skin, while in common commercial use, the seeds often are pressed to release the juice and served in cafes and restaurants as a centrifuged drink, but nowadays ample choice is given to consumers, with commercial pomegranate products such as canned beverages, jelly, jam, paste, and even vinegars. Pomegranate juice finds also use for flavouring and colouring beverage products [1]. Moreover, the need of methods for the characterization of food matrices chemical composition with the aim to assess the presence of healthy nutrients and bioactive compounds has gained great importance, also among consumers. These needs led to the development of analytical approaches for a comprehensive assessment of the benefits and risks associated with food intake [12]. Thus, in last years, metabolomics quickly emerged as a trustable and effective tool in food and nutrition sciences as it uses an untargeted approach that takes into account the most abundant low-molecular weight compounds present in any biological matrix. Among the analytical techniques used, NMR spectroscopy has been demonstrated to be widely used for characterization of quality, origin, adulteration, etc., of food products, including many types of fruits [13,14] and fruit juices [15]. This high throughput, reliable, non-invasive, high and reproducible technique provides quantitative and structural information on either specific molecules or complex mixtures [13], with minimal sample manipulation (typically just buffer addition) [15]. Thus, together with High Performance Liquid Chromatography coupled to Mass Spectroscopy (HPLC-MS), Nuclear Magnetic Resonance Spectroscopy (NMR) is widely used in metabolomic applications for food science and nutrition research [[16], [17], [18]]. In particular, the metabolomic approach based on NMR spectroscopy, has proven to be a powerful and reliable tool to obtain a simultaneous multiple-metabolites snapshot of biological samples for biomarker detection, food quality control, and/or origin discrimination [16,[19], [20], [21], [22]].
There is a great number of studies on the composition of pomegranate juice, but very little of them are based on NMR spectroscopy [[23], [24], [25], [26], [27]]; moreover, to the best of our knowledge, there is no published study on the 1H NMR metabolomic analysis of pomegranate wines. Thus, few studies focused on the phytochemicals and qualitative characterization of pomegranate wines also related to their evolution during the fermentation processes by different yeast strains [[28], [29], [30]]. Similarly to the fresh fruits, pomegranate wine may show many health benefits. It contains antioxidants and anthocyanins in large amounts. These molecules are beneficial for anti-aging, cardiovascular diseases, kidney diseases, and diabetes (Fellipe Lopes de Oliveira et al., 2020; Kandylis & Kokkinomagoulos, 2020) [31,32]. Therefore, we decided to characterize the juice of the two cultivars used for the production of the wine, and the wine themselves, focusing on the changes in concentrations of biologically relevant molecules from juice to wine, and moreover on the impact of the yeasts used for the vinification on the final product. In particular we focused, for the first time, on the metabolic profile transformations of pomegranate fruit juice after fermentation with specific yeasts by 1H NMR spectroscopy associated to multivariate statistical analysis. Moreover, a panel test of the wines was organised to assess the organoleptic quality of the fermented beverages, and to allow correlation of the tasting parameters data with the chemical profiles provided by NMR spectroscopy, with the intent of establishing a chemical pathway to the choice of both cultivar and yeast in relation to the consumer perceptions. The obtained results allowed us to provide useful information for the selection of cultivar × yeast fermentation patterns able to provide a product with the best nutraceutical and organoleptic characteristics.
2. Materials and methods
2.1. Collection of pomegranate fruits and sample preparation
Pomegranate juice was obtained from freshly pressed fruits of the commercially available Smith and Jolly Red cultivars collected in fields belonging to the company ICS s.r.l., as previously described [33]. Two juice samples, one for each cultivar, were stored for further NMR analysis. Afterwards, pomegranate wine was prepared by fermenting the fruits with the commercial yeasts Saccharomyces cerevisiae (strain Clos) and Saccharomyces cerevisiae ex-bayanus (strain EC1118) (both Lallemand Inc., Montreal, QC, Canada), according to the previously reported procedure [33]. A total of 12 wines were produced separately by fermenting each of the 2 cultivars with each of the 2 yeasts, in 3 replications of winemaking. Wines were analyzed 3 months after fermentation end.
2.2. NMR sample preparation
Pomegranate juice and wine samples (900 μL each) were added with 100 μL of a phosphate buffer solution at pH 2.9. The buffer contained 1 M KH2PO4 in D2O, 0.1% trimethylsilylpropanoic acid (TSP) as internal reference standard, and 2 mM NaN3 to prevent microbial contamination. The resulting solution was successively centrifugated in order to remove solids (10,000 g at room temperature for 5 min). Then, 600 μL of the supernatant was transferred into a 5 mm NMR tube for spectral acquisition [13,34].
2.3. NMR experiments
NMR spectra were acquired on a Bruker Avance III 600 Ascend NMR spectrometer (Bruker, Germany) operating at 600.13 MHz for 1H observation, equipped with a z axis gradient coil and an automatic tuning-matching unit (ATM). The spectra were acquired at the temperature of 300 K with a Bruker Automatic Sample Changer, interfaced with the software IconNMR (Bruker). 1H 1D spectra were acquired using a standard pulse program including presaturation for residual solvent signal (zgcppr). 64 scans (with 16 dummy scans) were collected into 65536 data points, with a rcicle delay of 5.0 s. A spectral width of 12,019 Hz corresponding to 20.028 ppm (acquisition time of 2.7262144 s) were used. The resulting FIDs were weighted with an exponential function (line broadening of 0.3 Hz) before Fourier transform, phasing, and baseline correction. Spectra were referenced setting the trimethyl silyl propionate standard signal at 0.00 ppm. Spectra were processed with TopSpin 3.5 pl 7 software (Bruker). Homo- and hetero-correlated 2D NMR spectra, using standard pulse sequences, were used to assign the metabolites (2D 1H Jres, 1H COSY, [1H,13C]-HSQC, and [1H,13C]-HMBC). Assignments were confirmed by comparison with literature [13,[24], [25], [26]].
The acquisition and processing of 2D spectra were performed as follows:
2D [1H,1H]-COSY spectra, pulse sequence with presaturation during relaxation delay using gradient pulses for coherence selection, spectral width in both dimensions 12,019 Hz (20.028 ppm), 2048 data points in f2, 512 increments in f1, processed with a-bell squared window function in both dimension before Fourier transform;
1H homonuclear J-resolved, spectral width 12 kHz for f2 dimension and 80 Hz for f1, 8096 data points in f2, 256 increments in f1, processed with zero filling in f1 to 4096 real data points, unshifted sine-bell squared window functions in both dimensions before Fourier transform;
[1H,13C]-HSQC,1H–13C decoupling, 9 and 37 kHz spectral widths in the 1H and 13C dimensions respectively, 2048 data points in f2, 256 increments in f1, forward Linear Prediction with 32 coefficients, zero filling to 4096 data points for the f1 dimension, unshifted sine-bell squared window functions were applied in both dimensions before Fourier transform.;
[1H,13C]-HMBC, 9 and 45 kHz spectral widths in the 1H and 13C dimensions respectively, 2048 data points in f2, 512 increments in f1, forward Linear Prediction with 32 coefficients, zero filling to 4096 data points in f1, unshifted sine-bell squared window functions in both dimensions dimension before Fourier transform.
2.4. NMR data processing and statistical analysis
1H NMR spectra were segmented in buckets of the same size (0.04 ppm width), and integrated using Bruker Amix 3.9.15 (Analysis of Mixture, Bruker BioSpin GmbH, Rheinstetten, Germany) software. The residual non-deuterated water (4.9–4.75 ppm) and ethanol (3.68–3.58 and 1.24–1.15 ppm) signals were excluded from the bucketing, performed within the 10.00–0.5 ppm region. Total sum normalization was applied [35] in order to reduce small differences due to metabolites concentration and/or small fluctuations of experimental conditions among samples. Pareto scaling method [35] was then applied to the bucket reduced NMR spectra. The data table obtained from all aligned and bucket-reduced spectra was then used for multivariate data analysis. For identification, each bucket was labelled with the value of the central chemical shift for its 0.04 ppm width. Simca-P version 14 program (Sartorius Stedim Biotech, Umeå, Sweden) was used to perform the following multivariate statistical analysis: unsupervised principal component analysis, PCA, and supervised orthogonal partial least squares discriminant analysis, OPLS-DA pattern recognition methods. The PCA was used in the first place to get an overview of all observations in the data table [8,9]. OPLS-DA was applied in order to highlight discriminating variable in the two class problems filtering the portion of the variance useful for predictive purposes from the non-predictive variance [36]. Statistical models were validated by an internal cross-validation method (7-fold) and a permutation test (40 total permutations) [11]. Models’ quality was assessed by evaluation of the total variation in X, the variation in the response variable Y, and the predictive ability of the models, described by the R2X, R2Y, and Q2 parameters, respectively [12]. The variables responsible for the observed discrimination were identified by using an S-line plot. Mean values ± standard deviation of selected and distinctive bucket reduced NMR signal areas (normalized to the total spectrum excluding the residual water region) were used to evaluate the relative change in discriminating metabolite content between the observed groups [16]. Log2 fold change (FC) ratio of the normalized median intensity of the corresponding signals in the spectra of the groups was used to assess changes in metabolite level between two groups [13,14]. Statistical significance was assumed for adjusted p-values <0.05. The Sparse PCA (SPCA) and the standard PCA used for the comparisons were performed with the package MixOmics [37] for the R Statistical Environment version 4.2.0 [38]. PCA biplots were also created with package MixOmics [37], while the correlation plot was created with the R corrplot package [39].
2.5. Sensorial analysis and correlation with chemical composition
On December 11th, 2020, pomegranate wines were tasted by 11 experts (including sommelier, oenologists, pomegranate producers, and wine researchers). All participants were of legal drinking age and were informed that the tasting involved alcoholic beverages. They were also informed that the study was an academic research project, that all data was going to be de-identified and only reported in the aggregate, that the information collected would be used for research purposes only, and that they could refuse to participate or stop participating in the study at any time without providing a reason. All participants agreed to an informed consent statement in order to participate to the study. The tasting sheet used for the description of the wine quality is available in supplementary material S1. For each organoleptic descriptor, each taster was asked to judge the intensity of the perception in each wine. Then, the ranking was obtained after normalization based on the total variance of the perception of each taster for each parameter. Variables were tested for normal distribution using the Shapiro-Wilk test, and the significant effects of the factors “cultivar”, “yeast”, “cultivar × yeast”, “replication” and “tester” were assessed by ANOVA, with the software SPSS v. 20 (IBM Corporation, Armonk, US). SPSS was also used to calculate Spearman correlations between the organoleptic descriptors evaluated in this work and the volatile compounds, as obtained in a previous report for the same wine samples [33].
The sensorial parameters tested were correlated with the compounds identified by NMR analysis using Pearson's rho correlations at p < 0.05, in order to identify the compounds potentially responsible for the organoleptic properties of the wines.
3. Results and discussion
3.1. Peak assignment in 1H NMR spectra
3.1.1. Pomegranate juice
Visual inspection of the 600 MHz 1H NMR methanol-d4 pomegranate juice spectra revealed a complex pattern of signals due to the presence of different compounds, mainly sugars, amino acids, organic acids, and polyphenols (Fig. 1). Relative expansions of significant spectral regions, with main metabolites assignment, performed on the base of literature data [[23], [24], [25], [26]] and bidimensional experiments, are reported in Fig. 1a, b, and 1c and Figs. S2 and S3.
Fig. 1.
Representative 1H NMR (Nuclear Magnetic Resonance Spectroscopy) spectrum of a pomegranate juice sample. Expanded areas in of (a) (−0.5–3 ppm), aliphatic region; (b) (3–5 ppm) sugars region; (c) (5–10 ppm) aromatic region. Due to the weakness of their intensities, this spectral region was reported with a high peak intensities' enhancement. The peaks of relevant identified metabolites are labelled.
In the low frequency field region of the spectrum (0.5–3.00 ppm) the resonances of aliphatic groups of amino acids and organic acids were identified. In particular, for the amino acids class, valine, threonine, alanine, arginine, glutamine, GABA (γ-amminobutyrate), asparagine, and arginine were identified (Fig. 1a). Presence of alcohols such as ethanol was also detected. The most intense peaks in this region were ascribable to citrate, the predominant organic acids. A marked difference in concentration of citrate could be observed by simple superimposition of Jolly Red and Smith 1H NMR spectra (Supplementary Fig. S4).
Moreover, signals assigned to malate and lactate were also observed in the spectra. Intense peaks assigned to anomeric protons of sucrose, α,β-glucose, and fructose dominated the overlapping middle frequency region of the spectrum (range 3–6 ppm) (Fig. 1b). Minor signals assigned to myo-inositol were also detected. The high frequency region of the spectra (5.0–9.5 ppm) showed peaks assigned to phenolic compounds with also important health promoting properties [8] could be observed (Fig. 1c). Among these, aromatic protons resonances of ellagitannins punicalagin were observed. This was confirmed by the assignments in the 2D [1H,13C]-HSQC and [1H,13C]-HMBC spectra (Supplementary Figs. S2 and S3) and comparison with literature data [25]. These molecules were already investigated for their antibacterial, antitumor and inflammatory activities [40]. Signals of antioxidants molecules as gallic and ellagic acid were also identified. As already reported in literature [13], the two broad signals observed at 6.90 and 7.60 ppm were assigned to other polyphenol compounds. Signals assigned to anthocyanin pigments, responsible for the red colour of the juice were also observed [24]. Other metabolites, such as trigonelline, together with aromatic amino acid as phenylalanine and tyrosine were also identified. A summary of the detected metabolites and their relative abundance is reported in Table 1, for juices and wines according to cultivar.
Table 1.
Qualitative comparison of selected metabolites by cultivar and beverage type. +, metabolite present, ++ metabolite in high concentration, — metabolite undetectable, ± metabolite in low concentration. GABA, ɣ-ammino butyric acid. * only detected in the case of EC1118 Yeast.
| Metabolite | Juice (Jolly Red) | Juice (Smith) | Wine (Jolly Red) | Wine (Smith) |
|---|---|---|---|---|
| Acetate | – | – | ± | ± |
| alanine | + | + | + | + |
| anthocyanins | ± | ± | – | – |
| arginine | ± | ± | ± | ± |
| asparagine | ± | + | – | – |
| 2,3-butanediol | – | – | ±* | ±* |
| citrate | + | ++ | ++ | ++ |
| ethanol | ± | ± | ++ | ++ |
| formate | + | + | + | – |
| fructose | + | + | + | + |
| GABA | ± | ± | – | – |
| gallate | ± | – | + | ++ |
| glutamine | + | + | – | – |
| glycerol | – | – | + | + |
| iso-pentanol/iso-butanol | – | – | ± | ± |
| lactate | ± | ± | – | – |
| malate | ± | ± | ± | ± |
| phenylalanine | ± | ± | ± | ± |
| succinate | – | – | + | + |
| sucrose | + | + | – | – |
| trigonelline | ± | ± | ± | ± |
| valine | ± | + | – | – |
| α-glucose | + | + | – | – |
| β-glucose | ++ | ++ | ± | ± |
3.1.2. Pomegranate wines
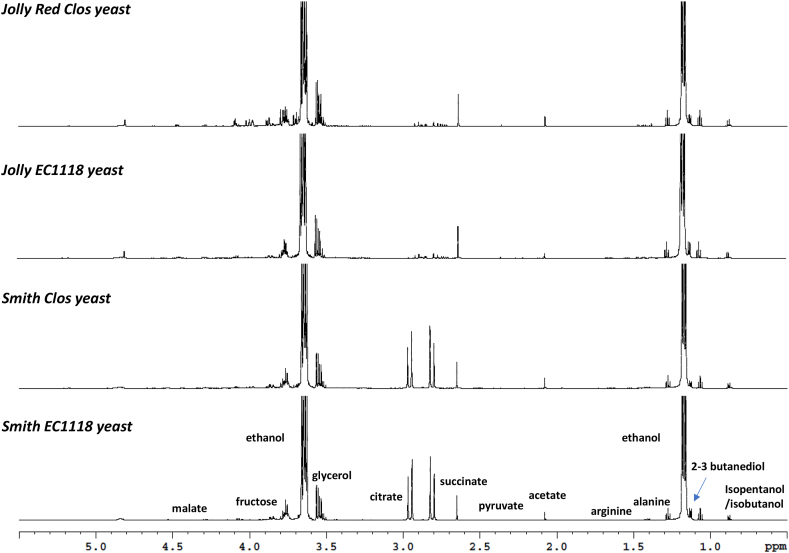
The 1H NMR aliphatic and aromatic region of the spectra of both Jolly Red and Smith cultivars’ fruit juice fermented by both Clos and EC1118 yeast were also characterized as reported in Fig. 2, Fig. 3, respectively.
Fig. 2.
Stacked plot of expanded area in the 0.5–5.5 ppm region of the 1H NMR (Nuclear Magnetic Resonance Spectroscopy) sample spectra of a fermented pomegranate juice sample. The peaks of identified metabolites are labelled accordingly.
Fig. 3.
Stacked plot of expanded area in the 6–9.5 ppm region of the 1H NMR (Nuclear Magnetic Resonance Spectroscopy) sample spectra of a fermented pomegranate juice sample. The peaks of identified metabolites are labelled accordingly.
As expected, the most intense signals in the spectra were assigned to ethanol (1.17 and 3.65 ppm), as an indicator of fermentation of sugars [41]. Moreover, signals of iso-pentanol/butanol and 2,3 butanediol [42] were identified. In the aliphatic region (0–3 ppm) proton resonances assigned to organic acids acetate, pyruvate, succinate, malate, and citrate were identified. Citrate seemed to remain almost constant after the fermentation, with similar values in both varietal juices and wines, as reported in literature data [43]. Interestingly, the same trend was observed for malic acid, the signal intensity of which remained similar in wines and did not exhibit noticeable changes during the winemaking process. The presence of organic acids is known to be strictly related to the organoleptic properties of wines [29]. In the middle frequency region of the spectra (3–6 ppm) the drastic reduction of glucose and sucrose signals could be observed as a consequence of fermentation. Moreover, as previous literature reported [29,30], while glucose is almost undetectable, fructose residues remains after the winemaking process as confirmed by the presence of the signals assigned to this reducing sugars. Likewise, signals assigned to the non-volatile alcohol glycerol were observed in all the fermented pomegranate varieties spectra. In the high frequencies region of the spectra the signal of gallate was observed with stronger intensity than that observed in fruit juice spectra as a consequence of ellagitannins hydrolysis and/or other oxidation reactions [8]. Likewise, proton resonances of flavonoids, 4-hydroxyphenylacetic acid and aromatic amino acids as phenylalanine and tyrosine were also identified. Signals of organic acid salts as formate and fumarate, and other metabolites as trigonelline could be also observed. Much lower intensities of anthocyanins signals were observed, as reported in literature. A decrease in the anthocyanins concentrations is an expected consequence of several processes taking place during winemaking [29]. A summary of the detected metabolites and their relative abundance is reported in Table 1, for juices and wines according to cultivar.
3.2. Multivariate statistical analysis: PCA and OPLS-DA
In order to reveal the possible data grouping of the samples, an unsupervised PCA was applied on the whole data set (Stacked plot of the spectral buckets reported in Supplementary Fig. S5). The resulting PCA model was built with 5 components giving R2X = 0.984, Q2 = 0.928, and showing a clear partition according to the pomegranate varieties along the t [1] principal component of the samples. Moreover, the cultivar Jolly Red showed an interesting discrimination along the t [2] component according to the yeast used for the fermentation. On the contrary, the cultivar Smith samples distributed as a quite compact group (Fig. 4).
Fig. 4.
PCA t [1]/t [2] scores plot for pomegranate wines data set. C: Clos yeast; EC: EC1118 yeast.
In order to specify the discriminating molecular components responsible for the observed clear discrimination, we refined the separation analysis between the two observed cluster, Jolly Red and Smith pomegranate cultivars, by pair-wise supervised OPLS–DA analysis. The resulting model is described by excellent parameters: 1 + 1+0 components gave R2X = 0.855, R2Y = 0.998 and Q2 = 0.997. Thus, the scores plot (Fig. 5a) for the model highlighted the discrimination among the two considered cultivars along the first component and, in this case, revealed the dispersion of Jolly Red cv. samples along to the orthogonal component. The corresponding S line plot (Fig. 5b) of the loading vectors for the first components, colored according the pcorr values, showed the molecular component that responsible for the observed separation among the samples. Higher relative value of citrate (binned at 2.98, 2.94 and 2.82 ppm) was observed as discriminating for the Smith cv. Moreover, for the Jolly Red variety, a higher relative content of fructose (binned at 4.1, 4.02, 3.9 ppm) and glycerol (binned at 3.74, 3.54, 3.58 ppm) and 2,3 butanediol (binned at 1.14 ppm) was observed.
Fig. 5.
(a) OPLS–DA t [1]/t [2] scores plot (t [1]/t [2] for Jolly Red and Smith pomegranates cultivar. (b) S line plot for the model colored according to the correlation-scaled coefficient (*p(corr) ≥|0.5|). C: Clos yeast; EC: EC1118 yeast. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The quantitative comparison between Jolly Red and Smith cultivar for the pomegranate discriminant metabolites was then performed by considering the fold change (FC) ratio [13,14], as reported in Fig. 6.
Fig. 6.
Discriminant metabolite comparison between Jolly Red and Smith pomegranate wine samples. The metabolites with significant Log2(FC) values are indicated with * (p ≤ 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In Smith pomegranate wine samples, a statistically higher content of citrate was observed. On the contrary, a statistically significant higher content of fructose, malate, glycerol and 2,3 butanediol was observed in Jolly Red pomegranate wines samples.
In order to characterize the metabolites with distinctive signals in the aromatic region, the unsupervised PCA analyses was also performed discarding the aliphatic region of the spectra (middle and low frequencies, from 6 to 0 ppm), obtaining an excellent model (five components, R2X (cum) = 0.996, Q2(cum) = 0.974) (Fig. 7). A certain degree of separation of the pomegranate varieties was observed in the t [1]/t [2] PCA scores plot, with Smith Clos and EC1118 pomegranate wines occurring at positive values of t [1] component, well differentiated Jolly Red Clos and EC1118 that placed from −0.3 to 0.2 values of t [1] component and at negative values of t [2] component.
Fig. 7.
PCA t [1]/t [2] scores plot for pomegranate wines data set in the aromatic region. C: Clos yeast; EC: EC1118 yeast.
Also in this case, a supervised discriminant analysis was performed in order to refine the separation among the groups and to identify the molecular component discriminating for the classes. The OPLS-DA analysis resulted in a good model descripted by 1 + 1+0 components giving R2X = 0.902, R2Y = 0.956 and Q2 = 0.916 (Fig. 8a). The S line plot (Fig. 8b) of the loading vectors for the model showed as variables at 7.14, ascribable to gallic acid signal is discriminating for the Smith varieties; on the contrary, signals ascribable to aromatic amino acid as tyrosine (binned at 7.22 and 6.94 ppm) and phenylalanine (binned at 7.38, 7.34 and 7.30 ppm) together with trigonelline (binned at 9.1, 8.82 ppm) were found as discriminating for the Smith variety.
Fig. 8.
a) OPLS-DA t [1]/t [2] scores plot for Jolly Red and Smith cultivar wines in the aromatic spectral region. b) S line plot for the model colored according to the correlation-scaled coefficient (*p(corr) ≥ |0.5|). C: Clos yeast; EC: EC1118 yeast. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The identified discriminant metabolites for Jolly Red and Smith cultivar pomegranate wines were then quantitatively compared by the FC ratio (Fig. 9) showing a general statistically significant higher content of gallate and alkaloid trigonelline, aromatic amino acids, 4-hydrophenylacetate for the Smith and Jolly Red cv. respectively.
Fig. 9.
Discriminant aromatic metabolite comparison between Jolly Red and Smith pomegranate wine samples. The metabolites with significant Log2(FC) values are indicated with * (p ≤ 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Multivariate statistical analysis: sparse PCA and interpretation of components
While PCA allows for an easy representation of the data in a dimensionally reduced space (see Fig. 4a), it is not easy to interpret the meaning of each of the principal components, given that the algorithm finds the new coordinates with the highest variance in order (Screeplot, Supplementary Fig. S6), but has no threshold on the weights of each variable constructing the principal components. This limitation reduces the number of variables to be considered but does not allow an easy correlation of the original variables to the new ones, and thus, a straightforward interpretation of the chemical meaning. Therefore, further actions have to be taken to evaluate the most important contributions. This limitation is not present in the sparse PCA methodology (SPCA), a variant of PCA that maximizes variance while at the same time setting to zero all the coefficients under a given threshold. The disadvantages of the SPCA over the classical PCA are the higher computational requirements and the need to optimise the number of coefficients for each PC in order to attain the maximum possible variance in the transformation. We performed the sparse PCA using the R package mixOmics [37] (implementing the regularized low rank matrix approximation algorithm by Shena and Huang [44] with a tuning procedure that chooses the optimal variance for each principal component number of variables selected) in order to pinpoint the NMR signals that had the maximum weight in discriminating the cultivar and the yeast strain in the final wines.
Before applying the sparse PCA (SPCA) algorithm, the data were subjected to Pareto scaling [35], as the packages provide only standard centering and autoscaling (Pareto scaling is the most common scaling used for NMR metabolomics data). For the tuning algorithm, up to a maximum of 10 variables (over a total of 230 buckets) for each of the 6 sparse principal components (as in standard PCA, 6 variables explain 99.38% of the cumulative variance) was allowed to determine the maximum variance explained, with 10 being selected as a reasonable upper limit for a straightforward interpretation of the contributions. Interestingly, the optimization procedure (Supplementary Fig. S7 outputs 1, 3, 3, 1, 3, and 1 variable as optimal for the six sparse principal components, with total explained variance of 32.05% (16.60, 5.60, 4.69, 4.10, 7.68, and 0.30% for the 6 components in order). Notwithstanding the much lower explained variance of the first two PC as compared to the standard PCA, the plot (Fig. 10) had a very simple interpretation: one of the buckets representing the citrate concentration was sufficient to discriminate between the cultivars used to obtain the wine on the principal component 1, with a relatively large separation among the single samples, while the information allowing to discriminate the yeast used was contained in the second sparse principal component, with 2,3-butanediol being a by-products of EC1118 and fructose of Clos. The loadings of the components were 1 for the Citrate.2 signal (at ∼2.82) in PC1, while PC2 had a 2,3-butanediol coefficient of 0.852, while Fructose.2 (the ∼3.9 ppm signal) and Fructose.6 (signal at ∼3.6 ppm) had loadings of −0.398 and −0.340, respectively. The signal dispersion seemed to indicate that the choice of the yeast had a reduced effect in the case of the Smith when compared to the Jolly Red cultivar.
Fig. 10.
Biplot of the sparse PCA showing the first two sparse principal components. On the axes in parenthesis, the percentage of explained variance. Light blue dots, samples obtained from the Jolly Red cv.; Orange dot, samples obtained from the Smith cv. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The plots obtained by the sparse PCA loadings (Supplementary Table S8) are very similar to those obtained by the standard PCA, and moreover, the results agree with those obtained by calculating the fold change in OPLS-DA, with an even simpler description of the key differences among the wines.
3.4. Sensorial analysis and correlation with chemical composition
Any significant effect for any of the considered parameters (see Supplementary Materials S1, Report sheet) was recorded for both the tasters and the wine replications, demonstrating a significant role of the cultivar, the yeast, and the cultivar × yeast interaction in the pomegranate wine organoleptic properties.
Jolly Red and Smith pomegranates were characterized by very different fruit quality. In fact, the cultivar significantly affected all the considered parameters except floral and herbal smells, the sapidity and the wine persistence (Table 2). Jolly Red obtained significantly higher rankings for most of the analyzed parameters: limpidity, intensity (olfactory), fruity, spicy, quality (olfactory), alcoholicity, softness, bitterness, balance, structure, quality (general) and harmony (Fig. 11a and b). On the other hand, Smith was characterized by a significantly higher perception of tonality, intensity (visual), acidity and astringency (Fig. 11c and d).
Table 2.
Significance of the effects of pomegranate cultivar, yeast, and cultivar × yeast interaction on the sensorial parameters tested. Significant effects and significantly affected descriptors are in bold.
| SENSORIAL PARAMETER | STATISTICAL SIGNIFICANCE (p) |
||
|---|---|---|---|
| CULTIVAR | YEAST | CULTIVAR × YEAST | |
| Limpidity | 0.000 | 0.214 | 0.016 |
| Tonality | 0.000 | 0.014 | 0.037 |
| Intensity - Visual | 0.000 | 0.396 | 0.447 |
| Intensity - olfactory | 0.000 | 0.826 | 0.621 |
| Fruity | 0.001 | 0.031 | 0.244 |
| Floral | 0.186 | 0.969 | 0.816 |
| Herbal | 0.281 | 0.390 | 0.137 |
| Spicy | 0.018 | 0.453 | 0.234 |
| Quality - olfactory | 0.000 | 0.178 | 0.198 |
| Alcoholicity | 0.000 | 0.045 | 0.440 |
| Softness | 0.000 | 0.605 | 0.569 |
| Sweetness | 0.000 | 0.000 | 0.000 |
| Bitterness | 0.043 | 0.266 | 0.002 |
| Acidity | 0.000 | 0.001 | 0.118 |
| Sapidity | 0.335 | 0.258 | 0.781 |
| Astringency | 0.000 | 0.184 | 0.221 |
| Balance | 0.000 | 0.438 | 0.992 |
| Structure | 0.001 | 0.001 | 0.014 |
| Persistence | 0.091 | 0.702 | 0.820 |
| Quality - general | 0.000 | 0.096 | 0.368 |
| Harmony | 0.000 | 0.870 | 0.888 |
Fig. 11.
Results of the sensory analysis of the pomegranate wines divided by cultivar and yeast. In figures a, b, c and d, light lines indicate the single wine profiles (3 replication reported) and dark line indicates the average of the 3 replications of each cultivar × yeast combination. In figure e, the radar chart showing the comparison among the cultivar × yeast combination averages.
The yeast used in the inoculum also significantly affected the wine perception (Table 2). Higher ranking in pomegranate wines were recorded in 2 parameters (fruity and acidity), and in 4 parameters (tonality, alcoholicity, sweetness, and structure) for wines fermented by EC 1118 and Clos respectively (Fig. 11e).
The differences in the sensory perception of pomegranate wines should be ascribed to their chemical composition.
In total, 170 negative and 243 statistically significant positive correlations (p < 0.05) were found between 21 sensorial properties tested and 39 compounds identified by NMR (Fig. 12, Supplementary Table S9). Two groups of compounds showed a higher number of correlations, and they had an opposite trend: gallate and citrate were positively correlated with tonality, intensity-visual, acidity and astringency, and negatively correlated with limpidity, intensity-olfactory, fruity, floral, spicy, quality-olfactory, alcoholicity, softness, sweetness, balance, structure, quality-general, and harmony. On the contrary, formate, fructose, glycerol and malate showed the opposite trend (Fig. 12, Supplementary Table S8). Further compounds positively correlated with general quality and harmony were trigonelline, histidine, phenylalanine, tyrosine, succinate, pyruvate, alanine and iso-pentanol/butanol.
Fig. 12.
Correlation plot of organoleptic descriptors and compounds identified by NMR in pomegranate wines. Color intensity of positive (blue) and negative (red) ellipses represent the level of the correlation; the size of the ellipses indicates the data dispersion. Only correlations with p-value<0.05 are plotted. A total of 170 negative and 243 statistically significant positive correlations (p < 0.05) were found between 21 sensorial properties and 39 identified compounds. Correlations between Pearson's rho and corresponding p-values are reported in Table S9. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The results of the sensorial analysis were also studied in relation to the previously published data concerning the volatile compounds [33]. One-hundred positive correlations and 132 negative correlations were obtained (further details concerning the specific compounds are reported in Table S10).
The complexity of this framework should be ascribed to the fact that the sensory perception of wines is the result of a huge number of interactions, including the ones among the chemical compounds in the solution and the ones of the molecules with the human receptor neurons. Positive correlations between sensorial properties tested and chemical compounds could be relatively easily ascribed to specific molecules. For example, it is well known that the yeast volatile metabolites and by-products such as alcohols, acetates and C4–C8 π-fatty acid ethyl esters contribute to the fermentation bouquet [45] However, it is worth to notice that the elaboration of our data also produced a large number of negative correlations. In wines, molecules chemically interact between each other, producing synergistic and antagonistic effects [46]. Furthermore, in mammalian, the response of olfactory receptor neurons to mixtures is strongly non-additive. An important role is played by inhibitory mechanisms responsible of antagonistic interactions among odorants [47]. Antagonistic interactions among sensorial properties and chemical compounds could also be very important in the definition of a production strategy: for example, ingredients showing bitterness suppression could be used to decrease added sugar content while preserving consumer acceptability in chocolate milk [48].
3.5. Multivariate statistical analysis: PCA and sparse PCA of sensorial analysis data
The data were centered and autoscaled before submitting them to PCA and sparse PCA algorithms. Nine components were needed to explain more than 99% of the total variance in this case (percentage of explained variance for PC1-PC9, in order: 61.43, 15.24, 8.45, 4.85, 3.62, 2.06, 1.84. 1.12, and 0.78% respectively). There was again a clear difference between the cultivars, with the first component discriminating between cultivars, and the second component discriminating, albeit in a less outstanding way, for the yeast used. Even in the tasting, the change in yeast strain had a less pronounced effect on the wine obtained from the Smith cultivar than it did on the Jolly Red (Biplot in Fig. 13a). For the sparse PCA tuning algorithm, up to a maximum of 12 variables (over a total of 230 buckets) for 5 sparse principal components were used. In this case, the optimization procedure kept a significant number of variables (11, 4, 11, 6, 3 for components 1–5, respectively), with a reasonably high total explained variance of 72.09% (42.14, 9.73, 8.56, 8.43, and 3.23% for the 5 components in order).
Fig. 13.
Biplot of the PCA (a) and SPCA (b) showing the first two principal components. On the axes in parenthesis, the percentage of explained variance. Light blue dots, samples obtained from the Jolly Red cv.; orange dot, samples obtained from the Smith cv. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The sparse PCA highlighted the wine organoleptic characteristics that are typical of Jolly Red (Intensity–olfactory, Softness, Spicy, Sweetness, Harmony, Balance and General Quality) and Smith (Acidity, Intensity–Visual, and Tonality) (Biplot in Fig. 13b, Loadings of the sparse PCA in Table 3).
Table 3.
Sparse loadings for the sparse principal components of the tasting data.
| PC1 | PC2 | PC3 | PC4 | PC5 | |
|---|---|---|---|---|---|
| Limpidity | 0.000 | 0.000 | 0.227 | −0.491 | 0.000 |
| Tonality | 0.325 | 0.000 | 0.031 | 0.000 | 0.000 |
| Intensity - Visual | 0.307 | 0.000 | 0.034 | 0.000 | 0.000 |
| Intensity -Olfactory | −0.332 | 0.000 | 0.000 | 0.000 | 0.000 |
| Fruity | 0.000 | 0.000 | 0.105 | −0.129 | −0.936 |
| Floral | 0.000 | 0.000 | 0.000 | −0.390 | 0.000 |
| Herbal | 0.000 | −0.680 | 0.000 | 0.000 | 0.000 |
| Spicy | −0.035 | 0.000 | 0.367 | 0.000 | 0.000 |
| Quality -Olfactory | −0.194 | 0.000 | 0.220 | 0.000 | 0.000 |
| Alcoholicity | 0.000 | 0.000 | −0.510 | −0.717 | 0.066 |
| Softness | −0.380 | 0.000 | 0.000 | 0.000 | 0.000 |
| Sweetness | −0.121 | 0.021 | 0.000 | 0.000 | 0.000 |
| Bitterness | 0.000 | −0.550 | −0.162 | 0.000 | 0.000 |
| Acidity | 0.319 | 0.000 | 0.000 | −0.118 | 0.000 |
| Sapidity | 0.000 | −0.485 | 0.000 | 0.000 | 0.000 |
| Astringency | 0.000 | 0.000 | 0.102 | 0.000 | 0.000 |
| Balance | −0.376 | 0.000 | 0.000 | 0.000 | 0.000 |
| Structure | 0.000 | 0.000 | 0.353 | 0.000 | 0.347 |
| Persistence | 0.000 | 0.000 | 0.576 | −0.251 | 0.000 |
| Quality -General | −0.357 | 0.000 | 0.000 | 0.000 | 0.000 |
| Harmony | −0.349 | 0.000 | 0.000 | 0.000 | 0.000 |
While in the case of the chemical identity of the wines the sparse PCA was able to adequately discriminate the yeast used (along the PC2 in both cases), none of the components in the sparse PCA of the tasting data was able to single out the difference (data not shown), suggesting the effect of the yeast on the final product being more subtle and distributed on many parameters.
4. Conclusions
In this work, a 1H NMR metabolic profile analysis in combination with MVA was performed for the first time in order to characterize different pomegranate wines produced with a novel protocol. In particular, four different types of pomegranate wines, previously obtained by using two different cultivars and two different yeast starters, were studied here by analysing their chemical and organoleptic composition. The NMR analysis revealed a statistically significant, clear discrimination between the cultivars, characterized by different metabolites content (higher content of organic acids citrate, gallate and fructose, malate, glycerol, 2,3 butanediol, trigonelline, aromatic amino acids and 4-hydrophenylacetate was observed for the Smith and Jolly Red pomegranate cv. wines samples, respectively). Moreover, a smaller, yet sizeable and significant, discrimination between the yeasts used from the chemical point of view. Sensorial analysis showed that both the cultivar and the yeast had significant influence on the composition and the organoleptic properties of the wines. Significant correlations between the chemical composition and the organoleptic parameters were found. Moreover, sparse PCA allowed to identify a limited number of parameters that could be referred as cultivar-dependent. On the other hand, the technique could not reproduce the yeast-dependent separation of the sample observed in the second principal component of PCA. Although further analyses are needed to achieve a complete characterization of this product, the NMR-chemometric study here reported could be considered as a promising starting point to define specific organoleptic and/or nutritional properties for consumer acceptance of pomegranate wines in Western countries.
Author contribution statement
Chiara Roberta Girelli, Paride Papadia, Francesca Pagano, Pier Paolo Miglietta, Massimiliano Cardinale, Laura Rustioni: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research received no external funding.
Data availability statement
Additional data not included in article/supp. material/referenced in article will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Carlo De Benedittis and Ilario D'amato (Italy) for supporting this study by providing both the pomegranate fruits and a financial contribution. Marco Mascellani, Ilaria De Simone and AIS Lecce for wine tasting. Prof. Vito Michele Paradiso is acknowledged for contributing to the volatile compounds analysis. PP would like to thank Dr. Ebe C. Princigalli for helpful discussion on the historical significance of pomegranate wine. The Graphical abstract contains an edited photo (available on commons.wikimedia.org under a https://creativecommons.org/licenses/by-sa/4.0 licence) of the painting “Einblicke” (Insights), oil on canvas, by the Austrian artist Matthias Laurenz Gräff.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16774.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Viuda‐Martos M., Fernández‐López J., Pérez‐Álvarez J.A. Pomegranate and its many functional components as related to human health: a review. Compr. Rev. Food Sci. Food Saf. 2010;9:635–654. doi: 10.1111/j.1541-4337.2010.00131.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruis A.R. Pomegranate and the mediation of balance in early medicine. Gastronomica. 2015;15:22–33. doi: 10.1525/gfc.2015.15.1.22. [DOI] [Google Scholar]
- 3.Daniel Zohary, Pinhas Spiegel-Roy. Beginnings of fruit growing in the old world. Science. 1975;187:319–327. doi: 10.1126/science.187.4174.319. [DOI] [PubMed] [Google Scholar]
- 4.Barnard H., Dooley A.N., Areshian G., Gasparyan B., Faull K.F. Chemical evidence for wine production around 4000 BCE in the Late Chalcolithic Near Eastern highlands. J. Archaeol. Sci. 2011;38:977–984. doi: 10.1016/j.jas.2010.11.012. [DOI] [Google Scholar]
- 5.Ward C. Pomegranates in eastern mediterranean contexts during the late Bronze age. World Archaeol. 2003;34:529–541. [Google Scholar]
- 6.Stover E., Mercure E.W. The pomegranate: a new look at the fruit of paradise. HortScience Horts. 2007;42:1088–1092. doi: 10.21273/HORTSCI.42.5.1088. [DOI] [Google Scholar]
- 7.Pliny the E., Rackham H. W. Heinemann; London: 1938. Natural History.https://www.biodiversitylibrary.org/item/74020 [Google Scholar]
- 8.Gumienna M., Szwengiel A., Górna B. European Food Research and Technology; 2016. Bioactive Components of Pomegranate Fruit and Their Transformation by Fermentation Processes; p. 242. [DOI] [Google Scholar]
- 9.Ferrara G., Cavoski I., Pacifico A., Tedone L., Mondelli D. Morpho-pomological and chemical characterization of pomegranate (Punica granatum L.) genotypes in Apulia region, Southeastern Italy. Sci. Hortic. 2011;130:599–606. doi: 10.1016/j.scienta.2011.08.016. [DOI] [Google Scholar]
- 10.Seeram N.P., Henning S.M., Zhang Y., Suchard M., Li Z., Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J. Nutr. 2006;136:2481–2485. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 11.Akhtar S., Ismail T., Layla A. In: Bioactive Molecules in Food. Mérillon J.-M., Ramawat K.G., editors. Springer International Publishing; Cham: 2019. Pomegranate bioactive molecules and health benefits; pp. 1253–1279. [DOI] [Google Scholar]
- 12.Hellberg R.S., DeWitt C.A.M., Morrissey M.T. Risk-benefit analysis of seafood consumption: a review. Compr. Rev. Food Sci. Food Saf. 2012;11:490–517. doi: 10.1111/j.1541-4337.2012.00200.x. [DOI] [Google Scholar]
- 13.Girelli C.R., De Pascali S.A., Del Coco L., Fanizzi F.P. Metabolic profile comparison of fruit juice from certified sweet cherry trees (Prunus avium L.) of Ferrovia and Giorgia cultivars: a preliminary study. Food Res. Int. 2016;90:281–287. doi: 10.1016/j.foodres.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Girelli C.R., Accogli R., Coco L.D., Angilè F., Bellis L.D., Fanizzi F.P. 1H-NMR-based metabolomic profiles of different sweet melon (Cucumis melo L.) Salento varieties: analysis and comparison. Food Res. Int. 2018;114:81–89. doi: 10.1016/j.foodres.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 15.Spraul M., Schütz B., Humpfer E., Mörtter M., Schäfer H., Koswig S., Rinke P. Mixture analysis by NMR as applied to fruit juice quality control. Magn. Reson. Chem. 2009;47 doi: 10.1002/mrc.2528. S130–S137. [DOI] [PubMed] [Google Scholar]
- 16.Girelli C.R., Serio F., Accogli R., Angilè F., De Donno A., Fanizzi F.P. First insight into nutraceutical properties of local Salento cichorium intybus varieties: NMR-based metabolomic approach. Int. J. Environ. Res. Publ. Health. 2021;18 doi: 10.3390/ijerph18084057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez-Casati D.F., Zanor M.I., Busi M.V. Metabolomics in plants and humans: applications in the prevention and diagnosis of diseases. BioMed Res. Int. 2013 doi: 10.1155/2013/792527. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobolev A.P., Mannina L., Proietti N., Carradori S., Daglia M., Giusti A.M., Antiochia R., Capitani D. Untargeted NMR-based methodology in the study of fruit metabolites. Molecules. 2015;20:4088–4108. doi: 10.3390/molecules20034088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobolev A.P., Segre A., Lamanna R. Proton high-field NMR study of tomato juice. Magn. Reson. Chem. 2003;41:237–245. doi: 10.1002/mrc.1176. [DOI] [Google Scholar]
- 20.Girelli C.R., Schiavone R., Vilella S., Fanizzi F.P. Salento honey (Apulia, south-east Italy): a preliminary characterization by 1H-NMR metabolomic fingerprinting. Sustainability. 2020;12 doi: 10.3390/su12125009. [DOI] [Google Scholar]
- 21.Savorani F., Rasmussen M.A., Mikkelsen M.S., Engelsen S.B. A primer to nutritional metabolomics by NMR spectroscopy and chemometrics. Food Res. Int. 2013;54:1131–1145. doi: 10.1016/j.foodres.2012.12.025. [DOI] [Google Scholar]
- 22.Girelli C.R., Del Coco L., Fanizzi F.P. 1H NMR spectroscopy and multivariate analysis as possible tool to assess cultivars, from specific geographical areas. EVOOs, European Journal of Lipid Science and Technology. 2016;118:1380–1388. doi: 10.1002/ejlt.201500401. [DOI] [Google Scholar]
- 23.Baldassarre F., Vergaro V., De Castro F., Biondo F., Suranna G.P., Papadia P., Fanizzi F.P., Rongai D., Ciccarella G. Enhanced bioactivity of pomegranate peel extract following controlled release from CaCO3 nanocrystals. Bioinorgan. Chem. Appl. 2022 doi: 10.1155/2022/6341298. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasanpour M., Saberi S., Iranshahi M. Metabolic profiling and untargeted 1H-NMR-Based metabolomics study of different Iranian pomegranate (Punica granatum) ecotypes. Planta Med. 2020;86:212–219. doi: 10.1055/a-1038-6592. [DOI] [PubMed] [Google Scholar]
- 25.Kraszni M., Marosi A., Larive C.K. NMR assignments and the acid–base characterization of the pomegranate ellagitannin punicalagin in the acidic pH-range. Anal. Bioanal. Chem. 2013;405:5807–5816. doi: 10.1007/s00216-013-6987-x. [DOI] [PubMed] [Google Scholar]
- 26.Tang F., Hatzakis E. NMR-based analysis of pomegranate juice using untargeted metabolomics coupled with nested and quantitative approaches. Anal. Chem. 2020;92:11177–11185. doi: 10.1021/acs.analchem.0c01553. [DOI] [PubMed] [Google Scholar]
- 27.Villa-Ruano N., Rosas-Bautista A., Rico-Arzate E., Cruz-Narvaez Y., Zepeda-Vallejo L.G., Lalaleo L., Hidalgo-Martínez D., Becerra-Martínez E. Study of nutritional quality of pomegranate (Punica granatum L.) juice using 1H NMR-based metabolomic approach: a comparison between conventionally and organically grown fruits. LWT. 2020;134 doi: 10.1016/j.lwt.2020.110222. [DOI] [Google Scholar]
- 28.Kokkinomagoulos E., Nikolaou A., Kourkoutas Y., Kandylis P. Evaluation of yeast strains for pomegranate alcoholic beverage production: effect on physicochemical characteristics, antioxidant activity, and aroma compounds. Microorganisms. 2020;8:1583. doi: 10.3390/microorganisms8101583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mena P., Gironés-Vilaplana A., Martí N., García-Viguera C. Pomegranate varietal wines: phytochemical composition and quality parameters. Food Chem. 2012;133:108–115. doi: 10.1016/j.foodchem.2011.12.079. [DOI] [Google Scholar]
- 30.Berenguer M., Vegara S., Barrajón E., Saura D., Valero M., Martí N. Physicochemical characterization of pomegranate wines fermented with three different Saccharomyces cerevisiae yeast strains. Food Chem. 2016;190:848–855. doi: 10.1016/j.foodchem.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 31.Kandylis P., Kokkinomagoulos E. Food applications and potential health benefits of pomegranate and its derivatives. Foods. 2020;9 doi: 10.3390/foods9020122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopes de Oliveira Fellipe, Portes Arruda Thaise Yanka, da Silva Lima Renan, Casarotti Sabrina Neves, Morzelle Maressa Caldeira. Pomegranate as a natural source of phenolic antioxidants: a review. J. Forensic Biomech. 2020;9 doi: 10.31665/JFB.2020.9214. [DOI] [Google Scholar]
- 33.Cardinale M., Trinchera R., Natrella G., Difonzo G., De Benedittis C., D’amato I., Mascellani M., Paradiso V.M., Rustioni L. Dynamics of the fermentation process and chemical profiling of pomegranate (Punica granatum L.) wines obtained by different Cultivar×Yeast combinations. Foods. 2021;10 doi: 10.3390/foods10081913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longobardi F., Ventrella A., Bianco A., Catucci L., Cafagna I., Gallo V., Mastrorilli P., Agostiano A. Non-targeted 1H NMR fingerprinting and multivariate statistical analyses for the characterisation of the geographical origin of Italian sweet cherries. Food Chem. 2013;141:3028–3033. doi: 10.1016/j.foodchem.2013.05.135. [DOI] [PubMed] [Google Scholar]
- 35.van den Berg R.A., Hoefsloot H.C., Westerhuis J.A., Smilde A.K., van der Werf M.J. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genom. 2006;7:142. doi: 10.1186/1471-2164-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eriksson L., Byrne T., Johansson E., Trygg J., Vikström C. Umetrics Academy; 2013. Multi- and Megavariate Data Analysis Basic Principles and Applications. [Google Scholar]
- 37.Rohart F., Gautier B., Singh A., Lê Cao K.-A. mixOmics: an R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017;13 doi: 10.1371/journal.pcbi.1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team R. R Foundation for Statistical Computing; Vienna, Austria: 2023. A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- 39.Wei T., Simko V. 2021. R Package “Corrplot”: Visualization of a Correlation Matrix.https://github.com/taiyun/corrplot [Google Scholar]
- 40.Lipińska L., Klewicka E., Sójka M. The structure, occurrence and biological activity of ellagitannins: a general review. Acta Sci Pol Technol Aliment. 2014;13:289–299. doi: 10.17306/j.afs.2014.3.7. [DOI] [PubMed] [Google Scholar]
- 41.Biais B., Allwood J.W., Deborde C., Xu Y., Maucourt M., Beauvoit B., Dunn W.B., Jacob D., Goodacre R., Rolin D., Moing A. 1H NMR, GC−EI-TOFMS, and data set correlation for fruit metabolomics: Application to spatial metabolite analysis in melon. Anal. Chem. 2009;81:2884–2894. doi: 10.1021/ac9001996. [DOI] [PubMed] [Google Scholar]
- 42.Consonni R., Cagliani L.R., Guantieri V., Simonato B. Identification of metabolic content of selected Amarone wine. Food Chem. 2011;129:693–699. doi: 10.1016/j.foodchem.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Mena P., Ascacio-Valdés J.A., Gironés-Vilaplana A., Del Rio D., Moreno D.A., García-Viguera C. Assessment of pomegranate wine lees as a valuable source for the recovery of (poly)phenolic compounds. Food Chem. 2014;145:327–334. doi: 10.1016/j.foodchem.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 44.Shen H., Huang J.Z. Sparse principal component analysis via regularized low rank matrix approximation. J. Multivariate Anal. 2008;99:1015–1034. doi: 10.1016/j.jmva.2007.06.007. [DOI] [Google Scholar]
- 45.Lambrechts M., Pretorius I. Yeast and its importance to wine aroma-a review. South Afr. J. Enol. Vitic. 2000;21:97–129. [Google Scholar]
- 46.Styger G., Prior B., Bauer F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011;38:1145. doi: 10.1007/s10295-011-1018-4. 1145. [DOI] [PubMed] [Google Scholar]
- 47.Reddy G., Zak J.D., Vergassola M., Murthy V.N. Antagonism in olfactory receptor neurons and its implications for the perception of odor mixtures. Elife. 2018;7 doi: 10.7554/eLife.34958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hopfer H., Riak A.C., Roberts R.F., Hayes J.E., Ziegler G.R. Synergistic and antagonistic ingredient interactions as a sugar reduction strategy in chocolate milk. J. Sensory Stud. 2022;37 doi: 10.1111/joss.12770. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data not included in article/supp. material/referenced in article will be made available on request.