Abstract
Two trials were performed to evaluate the association of hypothalamic abundances of thermosensitive transient receptor potential (TRP) ion channels with thermoregulation in broiler chickens. In trial 1, temporal changes in body temperatures, and hypothalamic expression patterns of TRP channels and thermoregulatory neurotransmitter concentrations were assessed from 3 to 42 d of age. In trial 2, the same variables were compared at 2 age stages between 2 distinct types of birds with high or low rectal temperatures (HRT or LRT). The core-to-brain temperature difference exhibited a rapid increase after hatching, arriving at a steady state in the fourth week (P < 0.01). The hypothalamus saw a progressive decrease of TRPV4 protein expression through 28 d (P < 0.01), followed by a great increase in the abundance of other channels right up to the end (P < 0.05). Compared to LRT birds, a decline in hypothalamic content of TRPV4 (P < 0.05), together with a bigger core-to-brain temperature difference (P < 0.01), was evident in the HRT counterpart at 33 d. In both trials, the core-to-brain and core-to-surface temperature differences were controlled in a synchronous and coordinated manner. These results allow concluding that developmental changes in the thermal sensitivity of hypothalamic neurons, determined by brain cooling capacity, involve a neuro-genomic mechanism, which regulates the ratio between thermosensitive TRP ion channels to attain a lower proportion of TRPV4 in comparison with other channels.
Key words: thermosensitive TRP ion channels, thermoregulation, brain cooling capacity, hypothalamus, broiler chickens
INTRODUCTION
Body temperature homeostasis is a matter of life and death, and requires precise regulation by the nervous system. The hypothalamus, especially the preoptic area (POA), serves as the principal thermostat that not only receives and integrates information from peripheral temperature sensors located in the skin, spinal cord and viscera, but also detects local brain temperature (Tb) changes with a strong impact on the core body temperature (Tc; Boulant, 2000; Morrison et al., 2008; Morrison and Nakamura, 2011). Modulation of the activity of warm sensitive neurons (WSNs), by directly cooling or heating of the POA, elicits heat conservation/thermogenesis or heat dissipation phenomena, respectively (Nakayama et al., 1961, 1963; Boulant, 2006). In WSNs, a primary mechanism of temperature sensitivity involves the prepotential which produces the action potential when reaching a threshold. Warming increases the prepotential's rate of depolarization, and this shortens the inter-spike interval and increases the firing rate (Griffin et al., 1996; Boulant, 1998). Among potential thermoregulatory neurotransmitters, prostaglandin E2 (PGE2)-induced cyclic AMP (cAMP) production can enhance the thermo-sensitivity of WSNs by its actions on the depolarizing prepotential (Griffin et al., 1990; Chow and Boulant, 1997), which may be a mechanism for endogenous factors to alter the activity of neurons that control thermoregulatory responses. The molecular basis of the thermal response of the prepotential, however, has remained elusive.
The discovery of thermosensitive transient receptor potential (TRP) ion channels, a subset of the TRP family, in cell membranes reveals a clear picture of the thermo-sensory reception. They allow an influx of cations, e.g. Ca2+, into the cell, altering membrane potentials (Nilius and Voets, 2005). The gating is extremely dependent on the temperature, showing very high or low Q10 values (changes in the current amplitude upon a temperature increase of 10 degrees; Clapham, 2003; Brauchi et al., 2006). All 6 known thermosensitive TRP channels perceive distinct temperature ranges. TRP vanilloid 1 (TRPV1) is activated by temperatures > 42°C, TRP vanilloid 2 (TRPV2) by > 52°C, TRP vanilloid 3 (TRPV3) by > 33°C, TRP vanilloid 4 (TRPV4) between 27°C and 42°C, TRP melastatin 8 (TRPM8) by < 25°C, and TRP ankyrin 1 (TRPA1) by < 17°C, when overexpressed in cultured cells (human embryonic kidney cells and Chinese hamster ovary cells) or Xenopus oocytes (Tominaga and Caterina, 2004; McKemy, 2005; Dhaka et al., 2006; Karashima et al., 2009). Since thermosensitive TRP ion channels are suggested to be involved in heat perception in peripheral tissues (Caterina, 2007; Vay et al., 2012), it is tempting to speculate that they mediate temperature sensation in a similar fashion in the hypothalamus.
So far, the knowledge about thermosensitive TRP channels is largely based on research in mammals. Little is known about other endotherms, such as birds. Broiler chickens have high metabolic rates, high body temperatures and poor heat dissipating abilities, making them an interesting model to study thermoregulation. The brain of the domestic fowl begins to differentiate as early as the second day of incubation, and continues to develop until 3 weeks posthatching (Rogers, 1995). During the early postnatal phase, the young chick has a limited capacity to generate heat in response to cooling (Tzschentke and Nichelmann, 1999; Nichelmann and Tzschentke, 2002). After entering the stage of full-blown homeothermy, chickens are able to maintain a stable body temperature, but are more vulnerable to the heat mainly due to their large size, intact plumage, and lack of sweat glands (Li et al., 2015). The thermoregulatory transition from poikilotherm to homeotherm is accompanied by the alteration in hypothalamic thermo-sensitivity, with a relatively high cold sensitivity in early life (Tzschentke and Basta., 2000, 2002). From the foregoing, it is hypothesized that this is a result of changes in the number and ratio of thermosensitive TRP ion channels located at the neuron membrane.
The aim of the present study was to investigate the relevance of thermosensitive TRP channels in the central nervous system to body temperatures of broiler chickens. Gene and protein expressions of hypothalamic thermosensitive TRPs were evaluated at different age stages and between individuals with high or low core body temperatures. To expose the underlying causal mechanisms, concentrations of some thermoregulatory neurotransmitters were also measured.
MATERIALS AND METHODS
All research procedures were approved by the Animal Care and Use Committee of Shandong Agricultural University (SDAUA-2020-017) and complied with the Regulations on the Administration of Laboratory Animals promulgated by National Science and Technology Commission of the People's Republic of China (Beijing).
Birds and Care
Day-old male hatchlings (Arbor Acres, Gallus gallus domesticus), purchased from Shandong Dabao Breeding and Processing Co., Ltd. (Xintai, Taian, Shandong, China), were reared in a 3-tier set of overlap cages (1.40 m × 0.70 m × 0.38 m) in an environmentally controlled chamber. Each cage was furnished with 1 pan feeder (25 cm diameter and 2.5 kg capacity) and 2 nipple drinkers, and contained 8 birds. The feeder space allowance amounted to 9.81 cm/bird, and the watering density came to 4 birds/drinker. Based on an effective area (excluding feeder space) of 0.93 m2 per cage, the stocking density was calculated as being 8.60 birds/m2. Artificial lighting was continuous before 2 d, and afterward a 23L (1:00–24:00 h):1D (0:00–1:00 h) schedule was applied. The light intensity was 20 lx until 7 d, 10 lx before 14 d, 5 lx by the end of 28 d, 3 lx from 29 to 38 d, and 2.5 lx up to 42 d. Manure was collected on polypropylene belts under each tier of cages and removed out of the house daily from one end.
All birds received a common starter (21.0% CP and 3000 kcal/kg of ME from 1 to 21 d) or grower (19.5% CP and 3100 kcal/kg of ME from 22 to 42 d) diet. Feed was provided ad libitum in pelleted form and water was available at all times. Eight birds (1 cage) were randomly selected to be weighed every week, and the mean BW was about 166 g at 7 d, 440 g at 14 d, 820 g at 21 d, 1280 g at 28 d, 1820 g at 35 d, and 2380 g at 42 d.
Thermal Environment Monitoring
The ambient temperature in the chamber was 33°C at 1 d, reduced by 1°C every other day to achieve 21°C at 25 d, then 20°C at 28 d, and was maintained as such thereafter. The RH was kept at 60 ± 5% throughout the experiment. They were simultaneously assessed using a digital thermo-hygrometer (HT-300, Instrutherm, São Paulo, Brazil). The wind speed remained nearly constant at around 0.5 m/s, which was measured with a hot wire anemometer (YK-2005AH, Lutron Electronic Enterprise Co., Ltd., Taipei, Taiwan). These measurement devices were installed at the geometrical center of the chamber where the birds were evaluated, and all meteorological data were recorded at regular intervals of 30 min from 06:00 to 18:00 h.
Surgery
To introduce a thermocouple into the brain, a minor craniotomy was made using the method of Arad (1991) with some modifications. In brief, chickens were anesthetized by inhaling a mixture of oxygen and isoflurane (1%–3%; Central Laboratory, Beijing Luhe Hospital, Capital Medical University, Beijing, China), then placed into a stereotaxic apparatus. The skull was exposed and a small hole (1 mm diameter) was drilled about 2 mm to the left of the midline, on the axis connecting the external ear openings. A guide tube made of a polyethylene catheter and the tip of a disposable syringe (1 mL, used as a flange) were implanted, so that the end of the former rested close to the hypothalamus and the latter was secured to the skull with dental cement. Subsequently, the guide tube was flushed with saline and capped, and the skin was sutured.
Birds were permitted at least 24 h postsurgery for recovery before measurements. The correct location of the intracranial guide tube was verified by postmortem examination in each experiment.
Experimental Design
Trial 1. A total of 120 broiler chicks (15 cages) were used. Starting at 1 d of age, 8 birds (1 cage) were randomly selected on a weekly basis (5, 12, 19, 26, 33 and 40 d), and subjected to the minimally invasive cranial surgery. Two days later, they had their body temperatures taken at 8:00 a.m. after a 12-h overnight fast, then were killed by exsanguination following CO2 stunning. The hypothalamus was harvested and immediately frozen in liquid nitrogen.
Trial 2. One-day-old and 30-day-old broiler chickens, 80 (10 cages) each of 2 age stages, were used. Rectal temperatures (RT) were taken individually under a fasting state early in the morning for 2 consecutive days to calculate the average value, and then 16 birds that had the highest (n = 8) or lowest (n = 8) RT were identified at each age stage. They underwent a surgery in the head, and had their body temperatures taken with an empty belly at 8:00 a.m. on the fourth day. After euthanasia, the hypothalamus was rapidly dissected and snap-frozen in liquid nitrogen.
Measurements
Body Temperatures
The bird was taken out of the cage and placed sideways on a small platform. The body surface temperature (Ts) was measured within less than 10 s using an infrared thermal camera (FLIR B335, FLIR Systems Inc., Wilsonville, OR) with a precision of ± 0.1°C and the spectrum of 7.5 to 13 µm. The camera was positioned at a distance of 1 m, and a height of 1 m from the bird, trying to acquire a complete image. The emissivity coefficient (ε) used was 0.94 for the feathered regions, and 0.95 for the featherless areas of the body (Nääs et al., 2010). From images of the broiler surface, a drawing tool in FLIR Thermocam Researcher Pro 2.10 (FLIR Systems) was used to delineate the comb, head, eye, ear, wattle, neck, back cape, flight feathers, wing bar, breast, thigh, leg/foot, drumstick, and tail. Mean temperatures of each zone were extracted from the software, and the average value was calculated.
Shortly after that, the rectal temperature was measured at a depth of 1 to 2.5 cm using a digital thermometer (HI98501, Hanna Instruments, Inc., Laval, Quebec, Canada) with a resolution of 0.1°C. Meanwhile, a welded copper-constantan thermocouple (0.12 mm wire diameter, coated with polyvinyl to a final diameter of 0.3 mm) was inserted into the brain guide tube. It was connected to an amplifier having a gain of 1000, the output of which was passed to a bucking voltage supply. Reference junctions were kept in water at a known temperature (measured with a standard mercury thermometer, ± 0.05°C) in an insulated bottle. Calibration charts were used to correct for changes in the reference junction temperature. The overall accuracy of the measuring system was ± 0.1°C. The whole measurement procedure took less than 1 min for each chicken.
The rectal temperature was regarded as an indicator of the core body temperature. The core-to-brain (Tc - Tb) and core-to-surface (Tc - Ts) temperature differences were calculated respectively with each recording.
ELISA Quantification
Hypothalamic PGE2 and cAMP concentrations were determined spectrophotometrically (ELx808 Absorbance Microplate Reader, Bio-Tek Instruments, Inc., Winooski, VT) with commercial ELISA kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China), as described by Tao et al. (2016) and Kong et al. (2017). In brief, the tissue was weighed and mechanically homogenized in 0.9% saline solution, then centrifuged at 3000 g for 10 min at 4°C. The supernatant was loaded onto ELISA plates in duplicate, and the manufacturer's instructions were followed. Total protein concentration of the homogenate was measured by bicinchoninic acid (BCA) assay (#P0012, Beyotime Biotechnology Co., Ltd., Shanghai, China) using bovine serum albumin as the standard. The levels of PGE2 and cAMP were expressed as units per mg protein.
RT-qPCR
Total RNA was extracted from the homogenized hypothalamus using TRIzol Reagent (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA), and portions (1 μg) were reverse-transcribed into sing-stranded cDNA with an oligo-dT primer using AMV Reverse Transcriptase (Takara Bio Inc., Otsu, Shiga, Japan) as previously described (Li et al., 2013). Real-time PCR was conducted on an ABI 7500 Fluorescent Quantitative PCR system (Applied Biosystems, Bedford, MA) with Fast SYBR Green Master Mix (Applied Biosystems) to monitor double-stranded DNA synthesis. Reaction conditions were as follows: 10 min at 95°C, followed by 40 cycles of 10 s at 95°C, 20 s at 60°C, and 30 s at 72°C. Using the comparative cycle threshold (CT) method (Livak and Schmittgen, 2001), the abundances of TRPV1, TRPV2, TRPV3, TRPV4, TRPA1 and TRPM8 transcripts were normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) within the sample, calculated as 2−ΔΔCT, and standardized with those at 3 d in trial 1 and those of LRT group at 4 d in trial 2 as the calibrator (assigned an expression level of 1).
Primer sequences (Table 1) were designed with software (Primer Express, v3.0.1, Applied Biosystems), and product identities were confirmed by melting curve analysis.
Table 1.
PCR primers of relevant genes used in this study.
| Gene1 | GenBank no. | Orientation | Primer sequence (5′→3′) | Product length, bp |
|---|---|---|---|---|
| TRPV1 | XM_046929895.1 | Forward | GATGGATCACCTGATGGCACCTTC | 105 |
| Reverse | TCAGAGCAGCCTGTGATGGAGTC | |||
| TRPV2 | XM_040687364.2 | Forward | AGCAACCAAAAGGACCCCAATCG | 91 |
| Reverse | CAAGTAAGCCATCCAGTGCCTCAG | |||
| TRPV3 | XM_040687357.2 | Forward | TCAATGCAGCATACACCGAAGAGG | 140 |
| Reverse | AAAGAAAATACCCTGGGCGTGAGC | |||
| TRPV4 | NM_204692.2 | Forward | AGCAAGATTGAGAACCGCCATGAG | 112 |
| Reverse | AGGAGACCACGCTGATGTAGAAGG | |||
| TRPA1 | NM_001318460.2 | Forward | CCGTTCCTGAGTTACACCGTTCTG | 89 |
| Reverse | TCCCCAACAGCCAAACCAATTAGC | |||
| TRPM8 | XM_046921262.1 | Forward | GCACCGCTGGGAGTGGATATTTC | 144 |
| Reverse | ACGCACAAGGGTTTGGACTCATTC | |||
| GADPH | NM_204305.2 | Forward | CTACACACGGACACTTCAAG | 244 |
| Reverse | ACAAACATGGGGGCATCAG |
TRP: transient receptor potential; TRPV1: TRP vanilloid 1; TRPV2: TRP vanilloid 2; TRPV3: TRP vanilloid 3; TRPV4: TRP vanilloid 4; TRPA1: TRP ankyrin 1; TRPM8: TRP melastatin 8; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Western Blotting
Frozen hypothalamus samples were homogenized, lysed and centrifuged at 12,000 g for 10 min at 4°C. The supernatant was collected, and the protein concentration was determined using a BCA kit (Beyotime). An equal amount (20 μg) of each protein sample was loaded onto 7.5% SDS-PAGE gels, transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA), and blocked with 5% non-fat dry milk in TBST. The membrane was then incubated with specific primary antibodies (1:1,000 dilution) overnight at 4°C: TRPV1 (#AF8250, rabbit polyclonal antibody from Beyotime), TRPV2 (#bs-10297R, rabbit polyclonal antibody from Beijing Biosynthesis Biotechnology Co., Ltd., China), TRPV3 (#BA2875-2, rabbit polyclonal antibody from Boster Biological Technology Co., Ltd., Wuhan, Hubei, China), TRPV4 (#AF8253, rabbit polyclonal antibody from Beyotime), TRPA1 (#AF8241, rabbit polyclonal antibody from Beyotime), TRPM8 (#PB0882, rabbit polyclonal antibody from Boster), and GAPDH (#AF0006, mouse monoclonal antibody from Beyotime). Subsequently, the corresponding secondary antibody (HRP-conjugated goat anti-rabbit or anti-mouse IgG, 1:1000; Beyotime) was added and incubated at room temperature for 4 h. Chemiluminescence was detected using the BeyoECL Plus kit (#P0018S, Beyotime). The band density was normalized to GAPDH, and presented as fold-change relative to that at 3 d in trial 1 and that of LRT group at 4 d in trial 2.
Statistical Analysis
Data were statistically analyzed by one-way ANOVA using the GLM procedure (SAS version 9.1, 2004; SAS Institute Inc., Cary, NC), with Tukey's test for multiple comparisons. The experimental unit was the individual bird, and mean differences were considered significant when P < 0.05.
RESULTS
Developmental Patterns of Body Temperatures
During the early posthatch period, the developmental trend was contrary between body surface temperature and either brain or rectal temperature (Figure 1). The former fell sharply (P < 0.01) from 37.0°C to 34.8°C in the second week, rebounded somewhat in the following week and then leveled off, whereas the latter 2 rose rapidly but allometrically (P < 0.01) from 38.3°C to 40.0°C and from 38.5°C to 41.0°C, respectively, for the first several days (3–7 d) and flattened out thereafter (despite that brain temperature was maximal at 21 d). Both core-to-surface and core-to-brain temperature differences increased continuously (P < 0.01) until 21 or 28 d, followed by a constant peak.
Figure 1.
Developmental changes in body temperatures of broiler chickens. Data represent means ± SEM of 8 birds per age stage. a-d Means without a common superscript differ significantly (P < 0.05).
Developmental Expression Patterns of Thermosensitive TRP Ion Channels in the Hypothalamus
Hypothalamic gene expressions of TRPV1, TRPV3, TRPV4, and TRPM8 showed little or no changes before 28 or 35 d of age (Figure 2A), but increased dramatically (P < 0.01) toward the end of the rearing period. Transcript levels of TRPV2 and TRPA1 remained relatively constant throughout, yet the former had a decline (P < 0.01) at 28 d.
Figure 2.
Developmental changes in mRNA (A) and protein (B) expressions of thermosensitive TRP ion channels in the hypothalamus of broiler chickens. Results are shown as fold changes after being standardized by GAPDH. Data represent means ± SEM of 8 birds per age stage. a-c Means without a common superscript differ significantly (P < 0.05).
Protein abundances of hypothalamic TRPV1, TRPV3, and TRPM8 stayed largely the same by the end of 28 d (Figure 2B), and then went up greatly (P < 0.05) in the finishing phase. The content of TRPV4 protein dropped markedly (P < 0.01) between 7 and 14 d of age, bottomed out until 28 d, and climbed up to the initial level (P < 0.01) within about 2 weeks. In both trials, protein expressions of TRPV2 and TRPA1 were not detected by Western blot analysis with commercially available antibodies used in this study.
Developmental Patterns of Thermoregulatory Neurotransmitters in the Hypothalamus
After a leveling off until 21 d, hypothalamic concentrations of PGE2 and cAMP had a huge surge (P < 0.01) from 28 to 42 d (Figure 3).
Figure 3.
Developmental changes in thermoregulatory neurotransmitter concentrations in the hypothalamus of broiler chickens. Data represent means ± SEM of 8 birds per age stage. a-c Means without a common superscript differ significantly (P < 0.05).
Comparison of Body Temperatures Between 2 Types of Birds
Rectal temperatures of the 2 types of birds were 40.3 ± 0.18°C vs. 41.2 ± 0.15°C at 4 d and 41.1 ± 0.19°C vs. 42.1 ± 0.19°C at 33 d, respectively (Figure 4). Compared with the LRT, HRT chickens had significantly higher (P < 0.01) brain and body surface temperatures at 4 d, and greater (P < 0.01) core-to-brain and core-to-surface temperature differences at 33 d.
Figure 4.
Body temperatures of 2 specially selected groups of broiler chickens. Data represent means ± SEM of 8 birds in each group per age stage. a, b Means without a common superscript differ significantly between the 2 types of birds (P < 0.05).
Differential Expressions of Thermosensitive TRP Ion Channels in the Hypothalamus Between 2 Types of Birds
At the age of 33 d, hypothalamic mRNA expression and protein abundance of TRPV4 were both decreased (P < 0.05) in HRT broilers relative to those with LRT (Figure 5).
Figure 5.
Hypothalamic mRNA (A) and protein (B) expressions of thermosensitive TRP ion channels in broiler chickens with high or low rectal temperatures. Results are shown as fold changes after being standardized by GAPDH. Data represent means ± SEM of 8 birds in each group per age stage. a, b Means without a common superscript differ significantly between the 2 types of birds (P < 0.05).
Content Comparison of Thermoregulatory Neurotransmitters in the Hypothalamus Between 2 Types of Birds
On d 4 of age, HRT birds had higher concentrations (P < 0.05) of hypothalamic PGE2 and cAMP than do LRT ones (Figure 6).
Figure 6.
Thermoregulatory neurotransmitter concentrations in the hypothalamus of broiler chickens with high or low rectal temperatures. Data represent means ± SEM of 8 birds in each group per age stage. a, b Means without a common superscript differ significantly between the 2 types of birds (P < 0.05).
DISCUSSION
Temporal Patterns of Body Temperatures
Chickens are precocial birds, whose thermoregulatory development ends up in full-blown homeothermy shortly after hatching (Tazawa et al., 1988; Nichelmann and Tzschentke, 2002). Using the relationship between various body temperatures and age, the developmental pattern of thermoregulation could be characterized in trail 1. Thus, a newly-hatched chick regulated its surface temperature at 36.7°C, core (rectal) temperature at 38.5°C, and brain temperature at 38.3°C. The first one decreased with age, whereas the last 2 increased at significantly different rates. They all appeared to achieve stability between 7 and 14 d, consistent with the previous report that the brain and cloacal temperatures of domestic chicken hatchlings increased as a power function of age and approached adult levels at around d 10 (Arad, 1991). A core-to-surface, but not core-to-brain, temperature difference had existed from the day of hatching. Both of them increased progressively with age and reached a plateau before 21 or 28 d, indicating the maximization of sensible heat dissipation efficiency and full maturity of brain cooling capacity during posthatching development (Arad et al., 1984b; Arad, 1991). Collectively, the present results show that fine adjustments of body temperatures (in terms of temperature gradients among the core, brain and surface) will occur after the fowl has progressed from a moderately hypothermic (although endothermic) state to almost complete homeothermy (as far as temperature values of the core, brain and surface are concerned).
Expression Patterns of TRP Channels in Relation to Brain Cooling
The ontogeny of brain cooling capacity, as reflected by the core-to-brain temperature difference, points to structural changes in the rete ophthalmicum (the avian extracranial heat exchanger; Arad et al., 1984a; Arad et al., 1987). Trial 1 of the present study suggested that a lower relative proportion of hypothalamic TRPV4 contributed to the developmental regulation of brain cooling as well. In the early posthatching phase, the gradually decreasing protein expression of TRPV4 was responsible for an improved brain cooling capacity, whereas at a later stage, the elevated abundance of other channels (causing an indirect drop in TRPV4 percentage) accounted for the ultimate ability of birds to cool the brain below core temperature. This view was supported by the reduced transcript and protein levels of TRPV4 in the hypothalamus of HRT birds at 33 d in trial 2, which had similar brain temperatures with the LRT, resulting in a larger core-to-brain temperature difference. While it is not known if TRP channels are direct temperature sensors in WSNs, the present study has obtained interesting observations correlating TRP channel expressions with hypothalamic thermoregulatory function.
The Biological Significance of Core-to-Brain Temperature Differences
The core-to-brain temperature difference is of unquestionable value in avoiding brain damage during core hyperthermia (Kilgore et al., 1976). In trial 1, the differential expression of hypothalamic thermosensitive TRP ion channels, along with the developmental capability of keeping the brain cooler than the deep-body, provided an explanation for the change of neuronal thermo-responsiveness in the PO/AH (preoptic area and anterior hypothalamus)-region from a high cold-sensitivity to a high warm-sensitivity in the transition from neonatal to adult phenotypes (Nakashima et al., 1987; Tzschentke and Basta, 2000). Namely, the bigger the core-to-brain temperature difference, the hotter the thermal sensation, and vice versa. The dynamics of core-to-brain temperature differences were matched by parallel alterations in the core-to-surface temperature gradient, whose enlargement with age to increase the sensible heat loss implied a diminished ability of birds to store heat. As sensible heat transfer becomes less efficient in hot conditions, the increased brain cooling in the older fowls may predispose body heat to evaporative dissipation. Likewise, the coordinated control of core-to-brain and core-to-surface temperature differences was observed between HRT and LRT chickens in trial 2, demonstrating that the degree of brain cooling rather than brain temperature per se is the determining factor for thermal sensation.
The Biological Significance of Brain Temperatures
During posthatching growth, especially after 21 d of age, a higher brain temperature was associated with a significant increase in the hypothalamic concentration of PGE2 and cAMP in trial 1, suggesting an elevation of thermoregulatory set-point (Revaz et al., 2006). Similar results were found with HRT birds at 4 d in trial 2, whose brain temperatures were also higher, leading to the same core-to-brain temperature difference as the LRT. The hypothalamic temperature has been shown to be involved in the osmoregulatory response in birds, and hypothalamic heating will bring about a saving of water (Simon-Oppermann et al., 1979). Therefore, it seems that regulation of hypothalamic temperature at a higher level during the grower-finisher period is part of an adaptation which enables the fowls to maintain water balance at a susceptible stage for evaporative heat dissipation.
Limitations of the Study
Although most birds appear to maintain their brain temperatures about 1°C below those of the body over a wide range of ambient temperatures (Arad et al., 1984a,b), the ontogenetic development of central control mechanisms in parallel with coordinated thermoregulatory responses to different thermal conditions may vary. This study was conducted at thermoneutrality, whose statements, to say the least, require confirmation in a hot or cold environment.
CONCLUSION
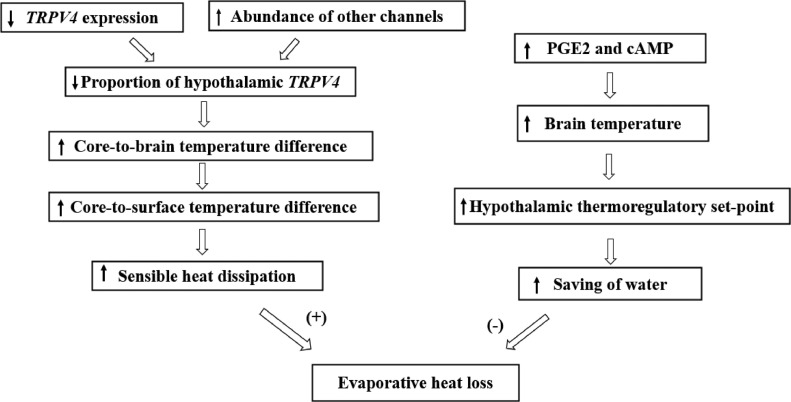
In summary, thermoregulation is mediated by the core-to-brain temperature difference in broiler chickens. The lower proportion of TRPV4 among hypothalamic thermosensitive TRP ion channels may represent a novel physiological mechanism for cooling the brain. The proposed mode is shown in Figure 7.
Figure 7.
Proposed model of posthatching ontogeny of brain cooling capacity and its association with thermoregulation in broiler chickens. ↑ssincrease; ↓ncdecrease; (+), stimulatory; (−), inhibitory.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (32072782, 31672442), National Key Research and Development Program of China (2021YFD1300405), Key Research and Development Program of Shandong Province (2019JZZY020602), “Taishan” Scholar Construction Project of Shandong Province (201511023), Funds of Shandong “Double Tops” Program and “Zhufeng” Talents Project of Shandong Agricultural University.
DISCLOSURES
All authors disclose not to have any actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations on the submitted work that could inappropriately influence, or be perceived to influence, their work.
REFERENCES
- Arad Z. Ontogeny of brain temperature regulation in chicks (Gallus gallus domesticus) Br. Poult. Sci. 1991;32:203–210. doi: 10.1080/00071669108417341. [DOI] [PubMed] [Google Scholar]
- Arad Z., Midtgard U., Berstein M.H. Post hatching development of the rete ophthalmicum in relation to brain temperature of Mallard ducks (Anas platyrhynchos) Am. J. Anat. 1987;179:137–142. doi: 10.1002/aja.1001790206. [DOI] [PubMed] [Google Scholar]
- Arad Z., Midtgard U., Skadhauge E. Effect of dehydration on body-to-brain temperature difference in heat-stressed fowl (Gallus domesticus) J. Comp. Physiol. B. 1984;154:295–300. [Google Scholar]
- Arad Z., Toledo C.S., Bernstein M.H. Development of brain temperature regulation in the hatchling Mallard duck (Anas platyrhynchos) Physiol. Zool. 1984;57:493–499. [Google Scholar]
- Boulant J.A. Hypothalamic neurons: mechanisms of sensitivity to temperature. Ann. NY Acad. Sci. 1998;856:108–115. doi: 10.1111/j.1749-6632.1998.tb08319.x. [DOI] [PubMed] [Google Scholar]
- Boulant J.A. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin. Infect. Dis. 2000;5:S157–S161. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- Boulant J.A. Neuronal basis of Hammel's model for set-point thermoregulation. J. Appl. Physiol. 2006;100:1347–1354. doi: 10.1152/japplphysiol.01064.2005. [DOI] [PubMed] [Google Scholar]
- Brauchi S., Orta G., Salazar M., Rosenmann E., Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J. Neurosci. 2006;26:4835–4840. doi: 10.1523/JNEUROSCI.5080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M.J. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R64–R76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- Chow A.R., Boulant J.A. Cyclic AMP effects on temperature sensitivity in rat hypothalamic neurons. FASEB J. 1997;11:A87. [Google Scholar]
- Clapham D.E. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Dhaka A., Viswanath V., Patapoutian A. Trp ion channels and temperature sensation. Annu. Rev. Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- Griffin J.D., Kaple M.L., Boulant J.A. Hypothalamic regional differences in neuronal responses to temperature and cyclic AMP. Soc. Neurosci. Abstr. 1990;16:574. [Google Scholar]
- Griffin J.D., Kaple M.L., Chow A.R., Boulant J.A. Cellular mechanisms for neuronal thermosensitivity in the rat hypothalamus. J. Physiol. (Lond.) 1996;492:231–242. doi: 10.1113/jphysiol.1996.sp021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y., Talavera K., Everaerts W., Janssens A., Kwan K.Y., Vennekens R., Nilius B., Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore D.L., Bernstein M.H., Jr, Hudson D.M. Brain temperature in birds. J. Comp. Physiol. B. 1976;110:209–215. [Google Scholar]
- Kong D., Zhang J., Hou X., Zhang S., Tan J., Chen Y., Yang W., Zeng J., Han Y., Liu X., Xu D., Cai R. Acetamiprid inhibits testosterone synthesis by affecting the mitochondrial function and cytoplasmic adenosine triphosphate production in rat Leydig cells. Biol. Reprod. 2017;96:254–265. doi: 10.1095/biolreprod.116.139550. [DOI] [PubMed] [Google Scholar]
- Li J., Chen Y., Wang Y.G., Zhao X.L., Gilbert E.R., Liu Y.P., Wang Y., Hu Y.D., Zhu Q. MUSTN1 mRNA abundance and protein localization is greatest in muscle tissues of Chinese meat-quality chickens. In. J. Mol. Sci. 2013;14:5545–5559. doi: 10.3390/ijms14035545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Wu J., Chen Z. Effects of heat stress on the daily behavior of Wenchang chickens. Braz. J. Poult. Sci. 2015;17:559–566. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McKemy D.D. How cold is it? TRPM8 and TRPА1 in the molecular logic of cold. Mol. Pain. 2005;1:16. doi: 10.1186/1744-8069-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.F., Nakamura K. Central neural pathways for thermoregulation. Front. Biosci. (Landmark Ed.) 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.F., Nakamura K., Madden C.J. Central control of thermogenesis in mammals. Exp. Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nääs I.A., Romanini C.E.B., Neves D.P., Nascimento G.R., Vercellino R.A. Broiler surface temperature distribution of 42 day old chickens. Sci. Agric. 2010;67:497–502. [Google Scholar]
- Nakashima T., Pierau F.K., Simon E., Hori T. Comparison between hypothalamic thermoresponsive neurons from duck and rat slices. Eur. J. Physiol. 1987;409:236–243. doi: 10.1007/BF00583471. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Eisenman J.S., Hardy J.D. Single unit activity of anterior hypothalamus during local heating. Science. 1961;134:560–561. doi: 10.1126/science.134.3478.560. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Hammel H.T., Hardy J.D., Eisenman J.S. Thermal stimulation of electrical activity of single units of the preoptic region. Am. J. Physiol. 1963;204:1122–1126. [Google Scholar]
- Nichelmann M., Tzschentke B. Ontogeny of thermoregulation in precocial birds. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002;131:751–763. doi: 10.1016/s1095-6433(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Nilius B., Voets T. TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflügers Arch. 2005;451:1–10. doi: 10.1007/s00424-005-1462-y. [DOI] [PubMed] [Google Scholar]
- Revaz S., Dudler J., Kai-Lik So A. Fever and musculoskeletal symptoms in an adult: differential diagnosis and management. Best Pract. Res. Clin. Rheumatol. 2006;20:641–651. doi: 10.1016/j.berh.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Rogers L.J. The Development of Brain and Behaviour in the Chicken. CAB International; Wallingford, UK: 1995. Development of the brain and behaviour before hatching. [Google Scholar]
- SAS Institute . SAS Institute Inc.; Cary, NC: 2004. SAS/STAT® 9.1 User's Guide. [Google Scholar]
- Simon-Oppermann C., Hammel H.T., Simon E. Hypothalamic temperature and osmoregulation in the Pekin duck. Pflügers Arch. 1979;378:213–221. doi: 10.1007/BF00592738. [DOI] [PubMed] [Google Scholar]
- Tao S., Han Z., Tian J., Cong R., Duanmu Y., Dong H., Ni Y., Zhao R. Downregulation of prostaglandin E2 is involved in hindgut mucosal damage in lactating goats fed a high-concentrate diet. Exp. Physiol. 2016;101:272–281. doi: 10.1113/EP085256. [DOI] [PubMed] [Google Scholar]
- Tominaga M., Caterina M.J. Thermosensation and pain. J. Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- Tazawa H., Wakayama H., Turner J.S., Paganell C.V. Metabolic compensation for gradual cooling in developing chick embryos. Comp. Biochem. Physiol. 1988;89A:125–129. [Google Scholar]
- Tzschentke B., Basta D. Development of hypothalamic neural thermosensitivity in birds during the perinatal period. J. Therm. Biol. 2000;25:119–123. [Google Scholar]
- Tzschentke B., Basta D. Early development of neural hypothalamic thermosensitivity in birds: influence of epigenetic temperature adaptation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002;131:825–832. doi: 10.1016/s1095-6433(02)00020-x. [DOI] [PubMed] [Google Scholar]
- Tzschentke B., Nichelmann M. Development of avian thermoregulatory system during the early postnatal period: development of avian set-point. Ornis Fennica. 1999;76:189–198. [Google Scholar]
- Vay L., Gu Ch., McNaughton P.A. The thermo-TRP ion channel family: properties and therapeutic implications. Br. J. Pharmacol. 2012;165:787–801. doi: 10.1111/j.1476-5381.2011.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]