Abstract

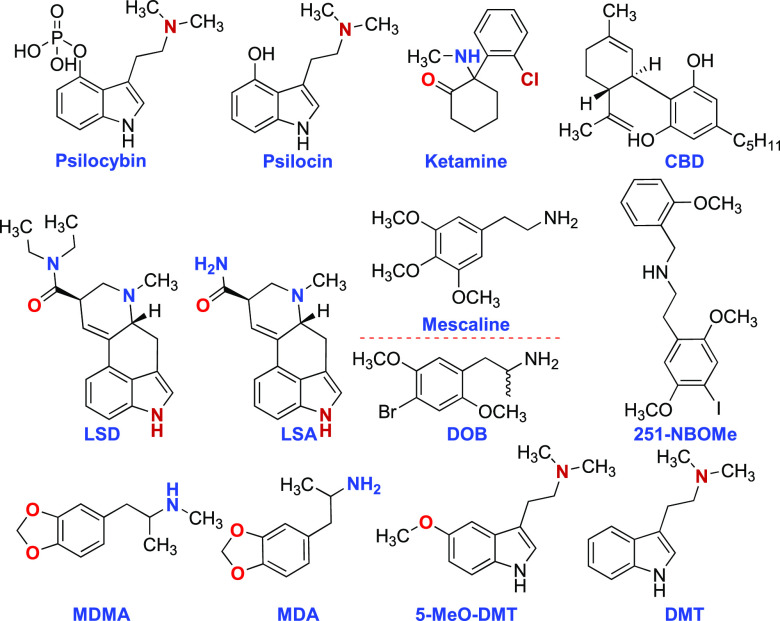
The gut–brain axis (GBA) refers to the sophisticated bidirectional communication system connecting the digestive system with the central nervous system. This interaction is enabled by a series of intricate signaling processes, encompassing various neuro-immune and hormonal pathways. The association between the gut microbiome and mental health has garnered immense scientific and public interest, driven by an enhanced understanding of the microbiome’s role in facilitating communication between the gut and the brain. This Patent Highlight discloses methods for promoting the colonization of spore-forming bacteria in the gastrointestinal track. These methods include administering a serotonin receptor agonist, such as psilocybin, psilocin, N,N-dimethyltryptamine, bufotenine, 5-methoxy-N,N-dimethyltryptamine, lysergic acid diethylamide, ergine, mescaline, 3,4-methylenedioxyamphetamine, 2,5-dimethoxy-4-methylamphetamine, and others.
Important Compound Classes

Title
Compounds and Methods for Modulating Lipid and Steroid Metabolism
Patent Publication Numbers
WO 2020/185581 A2 (URL: https://patents.google.com/patent/WO2020185581A2/en?oq=WO+2020%2f185581+A2)
Publication Dates
September 17, 2020
Priority Applications
US 62/815,760
Priority Dates
March 8, 2019
Inventors
Hsiao, E. Y.; Fung, T.
Assignee Company
The Regents of the University of California [US/US], 1111 Franklin Street, Twelfth Floor, Oakland, CA 94607-5200, United States
Disease Area
Neurological and psychological disorders
Biological Target
Gut microbiome
Summary
According to the National Institute of Mental Health (NIMH), in 2021, it was estimated that over 20% of U.S. adults were living with a mental illness (https://www.nimh.nih.gov/health/statistics/mental-illness, assessed 04/22/2023). The World Health Organization (WHO) reported that in 2019, one in eight individuals (970 million people) globally were affected by a mental disorder, with anxiety and depression disorders being the most prevalent (https://www.who.int/news-room/fact-sheets/detail/mental-disorders, assessed 4/22/2023). Considering the unmet needs, psychedelic medicine has emerged as one of the most rapidly expanding fields in medical research, experiencing unparalleled growth as an alternative approach to mental health treatment. Numerous studies propose that either the intensity and/or emotional aspect of the psychedelic experience can predict positive outcomes in conditions such as depression. However, this might simply reflect individual variations in brain biochemistry/physiology and receptor occupancy by the psychedelic, as higher receptor occupancy could potentially lead to more significant effects on both structural and functional neuroplasticity. The advancement of innovative human electrophysiological indices for studying brain plasticity is aiding in bridging the gap between pre-clinical and clinical understandings of psychedelic-induced modulations of plasticity.
Alternatively, neurochemical markers, such as brain-derived neurotrophic factor (BDNF)—a brain hormone that stimulates synaptic growth and plastic changes related to memory and learning—have been found to increase in human blood after psychedelic exposure. While most trial participants experience considerable benefits from a single treatment, like psilocybin treatment, there is a great deal of individual variation in the duration of effects, with some individuals potentially requiring multiple treatments to achieve lasting effects. Some research groups focus on studying the psychedelic experience or only minor experiential effects and, in some case, are developing analogues of psychedelics with antidepressant properties but without the “hallucinogenic” effect. The translational reliability of these various approaches in treating mental disorders remains relatively weak. However, studies involving healthy volunteers and patient populations in clinical trials may help resolve these uncertainties related to predictability, durability of effects, safety profiles, and efficacy.
The connection between the gut microbiome and mental health has attracted significant scientific and public attention, fueled by a growing understanding of the microbiome’s role in facilitating communication between the gut and the brain, known as the gut–brain axis (GBA). The GBA modulates the bidirectional interactions between the gut and the brain and plays a role in immunological, hormonal, and neural homeostasis. As a result, the gut microbiome influences the brain through various pathways, including the production of neuroactive metabolites, neurotransmitter synthesis, immune system activation, and vagus nerve stimulation.
The gut microbiota comprises an ecosystem of approximately 100 trillion microorganisms that engage in a symbiotic relationship with the host. Multiple factors known to affect the brain and psychiatric risk have been demonstrated to impact the gut microbiome, such as stress exposure, diet, and medications. Consequently, alterations in gut microbes can influence the central nervous system (CNS), including stress response, mood, and anxiety states. The 300–500 different species of bacteria possesses unique characteristics and abilities, including the synthesizing neurotransmitters. For example, Escherichia and Enterococcus have been known to produce serotonin (5-HT), while certain strains of Bifidobacterium and Lactobacillus produce γ-aminobutyric acid (GABA). Coprococcus and Faecalibacterium ferment non-digestible carbohydrates to create short-chain fatty acids (SCFAs) including acetate, butyrate, and propionate.
Additionally, SCFAs directly stimulate tryptophan hydroxylase, resulting in the synthesis of 5-HT, which is released from neurons in the enteric nervous system to regulate motility and appears to be a crucial mediator of the GBA. Notably, SCFAs can cross the blood–brain barrier (BBB), but GABA and serotonin typically cannot, except under inflammatory conditions that may alter the BBB permeability. Preclinical research has demonstrated that rodents exhibiting signs of depression and stress have abnormally low levels of SCFAs in their gut. The exact mechanism through which gut serotonin communicates with the brain has yet to be fully elucidated.
However, factors such as genes, age, sex, diet, life experiences, environment, and stress levels can all influence the development of the gut microbiota. Figure 1 illustrates the intricate relationship between microbiota, diet, and brain function throughout a lifetime.
Figure 1.

Schematic illustration of complex relationship between digestive system, diet, and brain function. [Schellekens, H.; et al. The microbiome-gut-brain axis in nutritional neuroscience. Nutritional Neuroscience2022, 2128007. Taylor & Francis Ltd., reprinted by permission of the publisher.]
Gut microbiota (GM)-derived metabolites can be classified into three categories based on their origin: (1) metabolites biosynthesized by GM, such as branched-chain amino acids, biogenic amines, histamine, and vitamins; (2) metabolites produced through the transformation of dietary components, including short-chain fatty acids, tyrosine, tryptophan catabolites, and trimethylamine-N-oxide; and (3) metabolites produced by the host and modified by GM, such as secondary bile acids. Translating this knowledge into clinical practice is challenging, partly due to each individual’s unique microbiota composition or the intricate fecal signature. The gut microbiota has emerged as a promising potential mechanism underlying individual differences in brain function, behavior, and psychiatric risk.
The patent application WO 2020/185581 A2 presents inventions based on select bacteria that are indigenous to the gut microbiota, which may bidirectionally signal with the host serotonergic system to promote their colonization and fitness in the intestine. There are studies that have demonstrated that increasing intestinal luminal serotonin levels through oral supplementation or genetic deficiency in the host serotonin transporter (SERT) enhances the relative abundance of spore-forming members of the gut microbiota. Turicibacter sanguinis is a gut bacterium that expresses a neurotransmitter sodium symporter (NSS)-related protein, which shares sequence and structural similarities with mammalian SERT. T. sanguinis imports serotonin through a mechanism that is inhibited by the selective serotonin reuptake inhibitor fluoxetine. Conversely, serotonin reduces the expression of sporulation factors and membrane transporters in T. sanguinis, an effect that is reversed by fluoxetine exposure. Gut microbiota association with T. sanguinis alters the intestinal expression of various gene pathways, including those crucial for lipid and steroid metabolism.
Furthermore, the patent disclosure provides methods for promoting colonization of spore-forming bacteria in a patient’s gut. Such methods include administering a serotonin receptor agonist orally, rectally, intravenously, intraperitoneally, or subcutaneously. Examples of the serotonin receptor agonist are serotonin, psilocybin, psilocin, N,N-dimethyltryptamine, bufotenine, 5-methoxy-N,N-dimethyltryptamine, lysergic acid diethylamide, ergine (LSA), mescaline, 3,4-methylenedioxyamphetamine, 2,5-dimethoxy-4-methylamphetamine and others. Spore-forming bacteria may downregulate the expression of certain genes, including Atf3, Bhlhe40, Bpgm, Ctss, Cldn4, Ceng2, Cdc42se2, Ccdc116, Dsg2, Encl, ENSMUSG00000104340, Fos, Fam84a, Fdftl, H2-BI, Hmgcs1, etc. Conversely, they may upregulate genes such as Acta2, Anxa6, Atg1612, Atp1b2, Ahrr, Banp, Ckm, Ckb, Creld1, Ddit4, Dmrt3, ENSMUSG00000102160, ENSMUSG00000108064, Fst, Flna, Gramdfa, Hsp90ab1, etc.
The regulation of these genes provides methods for treating or inhibiting an intestinal inflammatory disease by promoting colonization of spore-forming bacteria in a patient’s gut. Disease areas may include but are not limited to cardiovascular diseases, such as arteriosclerosis, atherosclerosis, stroke, ischemia, endothelium dysfunctions, peripheral vascular disease, cerebral infarction, myocardial infarction, coronary heart disease, restenosis, dyslipidemia, dyslipoproteinemia, or hypertension; neurodevelopmental disorders including autism spectrum disorder, Rett syndrome, fragile X, attention deficit disorder, and attention-deficit/hyperactivity disorder; and metabolic disorders such as type II diabetes, impaired glucose tolerance, insulin resistance, obesity, fatty liver, non-alcoholic steatohepatitis, or dyslipidemia.
Key Structures
Biological Assay
Colonization status in C57B1/6 and SERT–/– mice was monitored weekly by aerobic and anaerobic bacterial cfu plating and by 16S rDNA qPCR. APP+ update assay, [3H] 5-HT, [3H] NE, and [3H] Trip uptake assay, and statistical analysis were performed using Prism software (GraphPad).
Biological Data
The table below shows BLASTP alignment
scores between human (h) SERT with other mammalian biogenic amine
transporters, Turicibacter orthologs, and bacterial
amino acid transporter, LeuT.
Recent Review Articles
The author declares no competing financial interest.
This Patent Highlight published ASAP on May 11, 2023, with an error in the title. The corrected version was reposted on May 30, 2023.
References
- Majumdar A.; Siva Venkatesh I. P.; Basu A. Short-chain fatty acids in the microbiota-gut-brain axis: role in neurodegenerative disorders and viral infections. ACS Chem. Neurosci. 2023, 14, 1045–1062. 10.1021/acschemneuro.2c00803. [DOI] [PubMed] [Google Scholar]
- Yang D.; Huang W.; Wu C. W.; Huang C.; Yang Y. S.; Tung Y. Acute sleep deprivation exacerbates systemic inflammation and psychiatry disorders through gut microbiota dysbiosis and disruption of circadian rhythms. Microbiol. Res. 2023, 268, 127292. 10.1016/j.micres.2022.127292. [DOI] [PubMed] [Google Scholar]
- Lowe V. M.; Chaplin M.; Sgambato D. Major depressive disorder and the gut microbiome: what is the link?. Gen. Psychiatry 2023, 36, e100973. 10.1136/gpsych-2022-100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. S.; Fu C.; Gandhi T.; Fowler J. C.; Frueh B. C.; Weinstein B. L.; Petrosino J.; Hadden J. K.; Carlson M.; Coarfa C.; Madan A. Differential coexpression networks of the gut microbiota are associated with depression and anxiety treatment resistance among psychiatric inpatients. Progress Neuropsychopharmacol. Biol. Psychiatry 2023, 120, 110638. 10.1016/j.pnpbp.2022.110638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D.; Spriggs M.; Erritzoe D. Psychedelics therapeutics: What we know, what we think, and what we need to research. Neuropharmacology 2023, 223, 109257. 10.1016/j.neuropharm.2022.109257. [DOI] [PubMed] [Google Scholar]
- Schellekens H.; Ribeiro G.; Cuesta-Marti C.; Cryan J. F. The microbiome-gut-brain axis in nutritional neuroscience. Nutr. Neurosci. 2022, 2128007. 10.1080/1028415X.2022.2128007. [DOI] [PubMed] [Google Scholar]