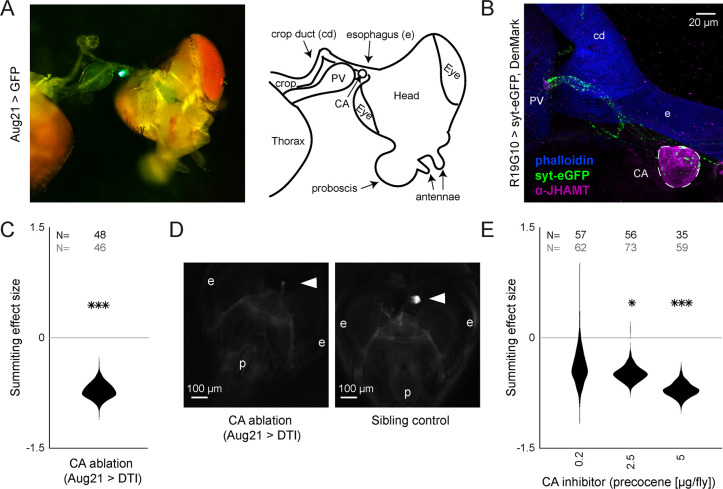
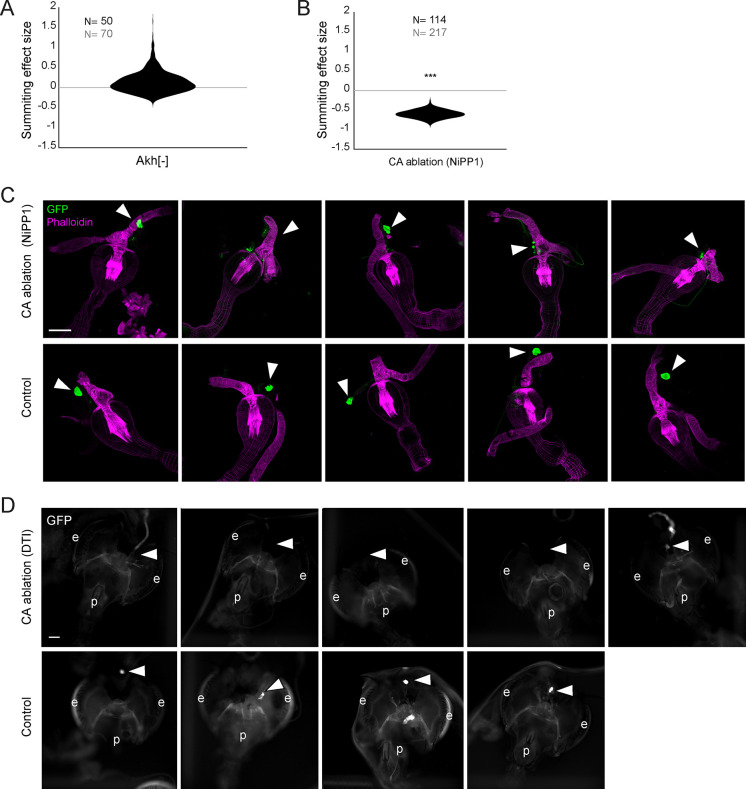
Figure 3. R19G10 (PI-CA) neurons project to the corpora allata, which are required for summiting behavior.
(A) Left: Composite micrograph of dissected Aug21>GFP fly, showing GFP fluorescence in the corpora allata (CA) overlaid on bright field image. Right: Diagram of A with anatomical features labeled. PV = proventriculus. (B) Representative confocal micrograph of immunostained RC from an R19G10>syt-eGFP, DenMark fly. Synaptic terminals are visible as green puncta, including in the CA. Magenta is anti-JHAMT and marks the CA. Blue phalloidin counterstain marks actin. Labels as in A. (C) Summiting effect size estimate distribution of ablating the CA with diphtheria toxin (DTI). Effect size is calculated relative to effector-less sibling controls. (D) Representative micrographs of CA-ablated and effector-less, sibling, temperature-matched control flies (additional examples in Figure 3—figure supplement 1D). White arrows indicate the expected location of CA. e = eye, p = proboscis. (E) Summiting effect size estimate distributions of various concentrations of the CA-ablating drug precocene. Effect size is calculated relative to vehicle (acetone) control. For (C and E), effect sizes were estimated as in Figure 2; asterisks indicate statistically significant effects (*=p<0.05; **=p<0.01; ***p<0.001) by two-tailed t-test. Sample sizes of experimental and control experiments are given in black and gray, respectively.