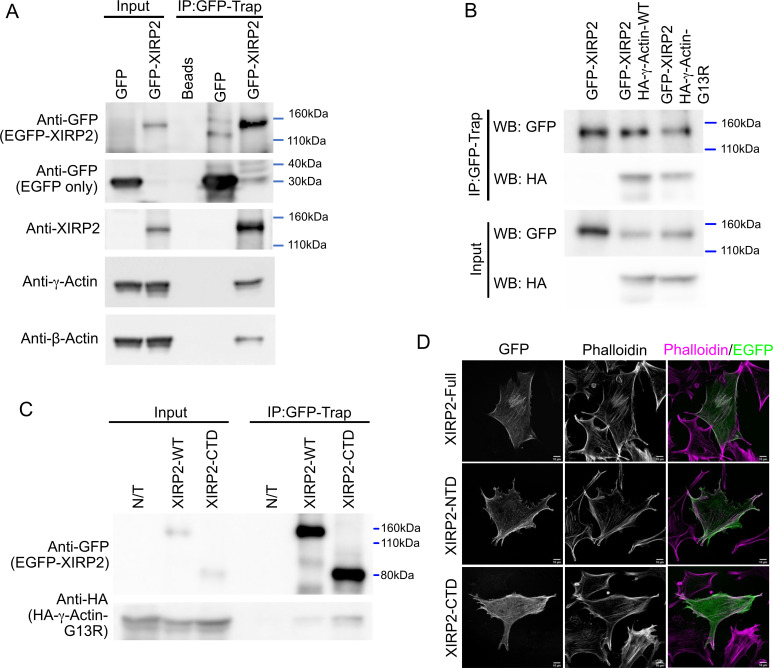
Figure 8. Xin actin binding repeat containing 2 (XIRP2) interacts with monomeric and filamentous actin through distinct domains.
(A) Endogenous β- and γ-actin co-immunoprecipitate with heterologously expressed short XIRP2. NIH 3T3 cells were transfected with the EGFP or EGFP-XIRP2 construct as indicated on the top of each lane. Total cell extract was loaded in input lanes. Immunoprecipitates (IP) were pulled down with GFP-Trap agarose beads followed by western blotting for the indicated antibodies (left). GFP-Trap beads (Beads) were incubated with non-transfected cell extracts as a negative control. (B) XIRP2 interacts with monomeric γ-actin. EGFP-XIRP2 WT plasmid was co-transfected with HA-γ-actin wild-type (WT) or the polymerization-incompetent HA-γ-actin G13R mutant. GFP-Trap beads were used for pulldown. Cells transfected with only EGFP-XIRP2 were used as negative control. EGFP-XIRP2 pulls down both the WT and the G13R mutant γ-actin. (C) The C-terminal domain (CTD) of XIRP2 is sufficient to pull down monomeric γ-actin. EGFP-XIRP2 WT or the CTD-encoding construct was co-transfected with HA-γ-actin-G13R plasmid. GFP-Trap beads were used for pulldown, and cells transfected with only HA-γ-actin-G13R (N/T) were used as negative control. Both the full-length and the CTD pulldown HA-γ-actin-G13R. (D) Full-length XIRP2 and the N-terminal domain (NTD) colocalize with F-actin, while the CTD is predominantly cytosolic and colocalizes only with a select subset of stress fibers. NIH 3T3 cells were transfected with plasmid constructs encoding EGFP-XIRP2 WT, NTD (including the LIM domain) or the CTD, fixed and counterstained with phalloidin.