Figure 1: Trial profile for CheckMate 914 part A.
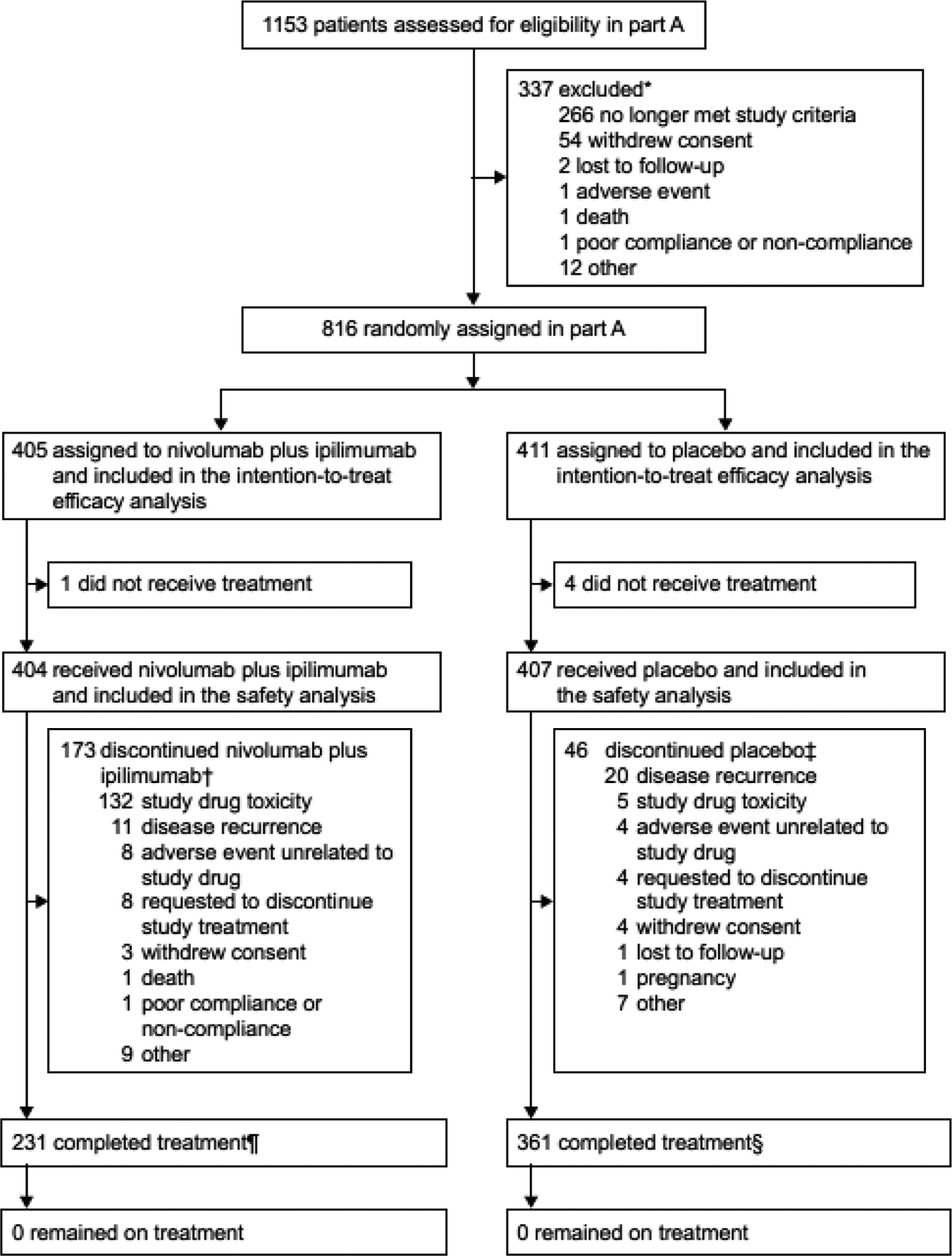
*Ten patients were not randomised due to COVID-19.
†Nine patients discontinued nivolumab plus ipilimumab due to COVID-19 (all nine patients discontinued due to “other” reason).
‡Eight patients discontinued placebo due to COVID-19 (one patient withdrew consent; seven patients discontinued due to “other” reason).
¶Two patients in the nivolumab plus ipilimumab arm were reported as having completed treatment by the investigators even though one patient skipped nivolumab at cycle 3 and one patient skipped nivolumab at cycle 12.
§One patient in the placebo arm was reported as having completed treatment by the investigator even though the patient skipped ipilimumab-placebo at cycle 7.
