Figure 2: Kaplan-Meier estimates of disease-free survival (primary definition*) per blinded independent central review.
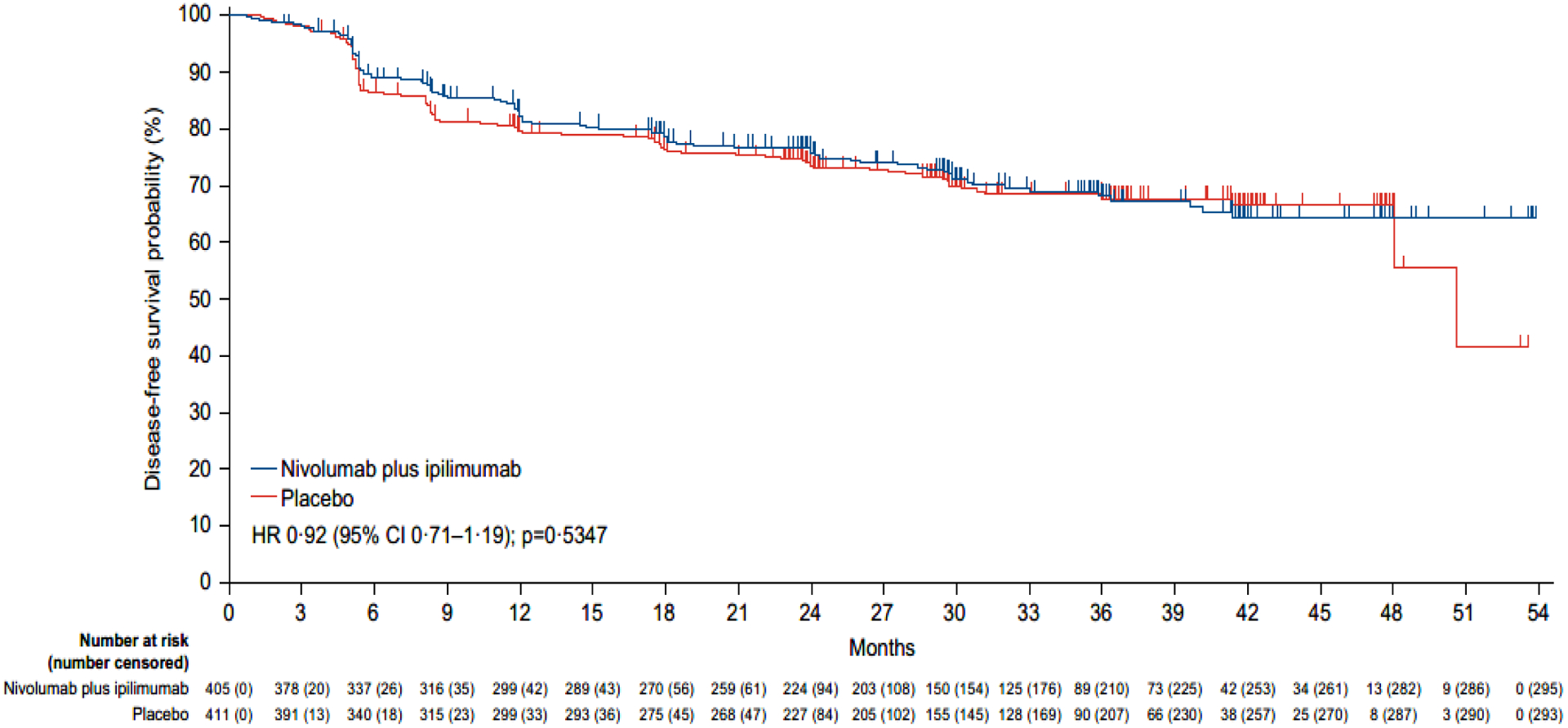
Disease-free survival according to blinded independent central review was assessed in all randomly assigned patients. The reported p value is two-sided. Tick marks represent data censored at the last time that the patient was known to be alive and free from disease recurrence.
*Subsequent therapy includes systemic anticancer therapy, tumour-directed radiotherapy, or tumour-directed surgery. Patients who died without a reported recurrence were considered to have recurred on the date of their death. The following censoring rules were applied for the primary definition of disease-free survival: 1. patients who did not recur or die were censored on the date of their last evaluable tumour assessment; 2. patients who did not have any on-study tumour assessments and did not die were censored on their date of randomisation; 3. patients who received subsequent systemic anticancer therapy, tumour-directed radiotherapy, or tumour-directed surgery before documented recurrence were censored at the date of the last tumour assessment conducted on or before the initiation of the new therapy; 4. patients who did not have a documented recurrence and received subsequent systemic anticancer therapy, tumour-directed radiotherapy, or tumour-directed surgery were censored at the date of the last tumour assessment conducted on or before the initiation of the new therapy (see supplemental methods for full censoring scheme).
