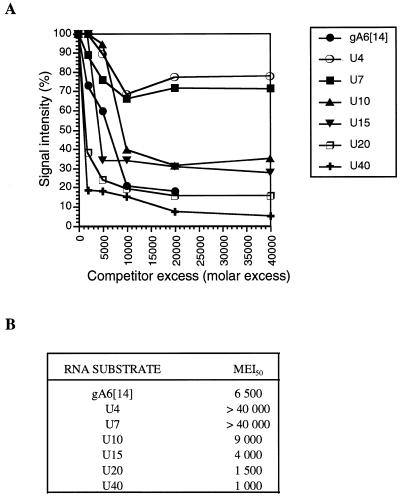
Figure 4.
(A) Determination of the RBP16 minimal binding site. Purified RBP16 (600 ng) was cross-linked to [32P]-labeled gA6[14] (5 fmol) in the absence of competitor or in the presence of increasing amounts of unlabeled competitor. The competitors were used at molar excesses ranging from 2000- to 40 000-fold as compared to the labeled gA6[14]. The cross-linked proteins were then separated on a 12.5% SDS–PAGE gel and visualized by autoradiography. The UV cross-linking signals were quantified by densitometry. (B) Molar excess required to achieve 50% inhibition of the RBP16 binding to [32P]-labeled gA6[14] (MEI50).