Abstract
Objective
Long-chain PUFA (LCPUFA) found in breast milk are derived from dietary sources and critical for optimal infant development. We examined associations between fish consumption and concentrations of LCPUFA and essential n-3 and n-6 fatty acids in breast milk among mothers living around Lake Victoria.
Design
We used cross-sectional analyses of associations between recent fish consumption and breast-milk fatty acid concentrations.
Setting
The study was conducted around Lake Victoria on Mfangano Island, Kenya, where multiple fish species are key dietary components and also are widely exported.
Subjects
Breast-feeding mothers (n 60) provided breast-milk samples, anthropometric measurements and questionnaire responses.
Results
In the previous 3 d, 97 % of women consumed a mean of 178 (sd 111) g fish (~2 servings/3 d). Mean breast-milk concentrations included DHA (0·75 % of total fatty acids), EPA (0·16 %), α-linolenic acid (ALA; 0·54 %), arachidonic acid (AA; 0·44 %) and linoleic acid (LA; 12·7 %). Breast-milk DHA concentrations exceeded the global average of 0·32 % in fifty-nine of sixty samples. We found native cichlids (Cichlidae) and dagaa (Rastrineobola argentea) contributed high levels of DHA, EPA and AA to local diets. We also found evidence for associations between fish species consumed and breast-milk LCPUFA concentrations when controlling for intake of other fish species, maternal body mass, maternal age, child age and exclusive breast-feeding.
Conclusions
The fatty acid composition of breast milk was influenced by the fish species consumed. Ensuring access to diverse fish and particularly inexpensive, locally available species, may be important for diet quality as well as infant growth and development.
Keywords: Dagaa, DHA, Fish consumption, Lake Victoria, Long-chain PUFA
Sufficient intake of long-chain PUFA (LCPUFA), including n-3 and n-6 fatty acids, is critical for infant growth and development( 1 ). LCPUFA include DHA, EPA and arachidonic acid (AA). The essential n-3 fatty acid linoleic acid (LA) is the parent of the n-6 fatty acid family, including AA, while the essential n-6 fatty acid α-linolenic acid (ALA) is the parent of the n-3 fatty acid family, including DHA and EPA( 2 ). DHA is essential for retinal development and DHA and AA are important components of neural membranes, while EPA and DHA have been associated with cardiovascular health and inflammation( 3 ). LCPUFA thus play important roles in brain and eye development, as well as in the immune, metabolic and automatic nervous systems( 3 ), and may also be important for immunological and cardiovascular health( 1 ). Supplementation and observational studies demonstrate links between LCPUFA and child neural development, attention and cognitive outcomes( 1 , 4 ).
Breast milk is the primary source of infants’ intake of LCPUFA. Yet breast-milk levels of LCPUFA can vary substantially, with up to tenfold variation in DHA across countries resulting from differences in dietary intake( 5 ). This substantial variation underscores the importance of access to high-quality foods rich in essential LCPUFA for pregnant and breast-feeding women.
Fish, and particularly fatty fish, are good sources of DHA and EPA. Maternal fish consumption is associated with higher concentrations of LCPUFA in breast milk( 6 – 8 ). Research has also shown a relationship between fish intake and breast-milk DHA in locations as diverse as the Philippines( 8 – 10 ), Iran( 11 ) and Sri Lanka( 12 ). Around Lake Malawi, for example, fatty acid content of breast milk exceeded global averages and infant plasma fatty acid levels were also high( 13 ). In Western populations, maternal fish intake has been linked to concentrations of breast-milk LCPUFA and children’s outcomes. Maternal fish consumption during pregnancy has been associated with higher child intelligence quotient at age 18 months( 14 ); maternal fish consumption during breast-feeding was also associated with higher child development scores at age 18 months( 15 ).
Despite links between fish intake and breast milk, little is known about the influence of different fish species on breast-milk levels of LCPUFA. While the fatty acid composition of breast milk is partially a result of long-term fat stores, variation can be substantial in response to fish consumption during the previous day( 6 ). Moreover, coldwater marine fish species have been documented as important to the LCPUFA supply( 16 ), but the relative value of tropical freshwater species has been less examined.
In many regions worldwide, fish are the primary source of income, high-quality food and LCPUFA( 17 , 18 ). However, even in areas where fishing livelihoods predominate, access to fish is complicated by dynamics involving gender, pricing and export patterns( 18 , 19 ). Consequently, fish access for local consumption may be restricted to specific fish species and often those that are lower in value. In low-income countries, women and infants may have less access to such high-quality foods and dietary LCPUFA may be limited( 20 ). Because of their international market value, nutritional content of export species is more often assessed, while the nutritional composition of non-market, native species that local people often rely on is mostly unknown.
In the present study, we assessed the association between recent consumption of the four most commonly consumed fish species (two native and two non-native) and the fatty acid profile of breast-feeding women in a cross-section of women living around Lake Victoria, Kenya. We also analysed the fatty acid composition of the two native fish species that contributed substantially to local diets and made comparisons with previous analyses of nutrient composition of non-native species.
Methods
Sample population
Sixty mothers and sixty-one infants (including one set of twins) were drawn from participants in a longitudinal cohort study of 303 households on Mfangano Island, Kenya. All enrolled women currently breast-feeding the participant child (n 11) or a younger sibling (n 49) were invited to join the sub-study. The cohort is comprised of randomly sampled households with a child <2 years of age and were enrolled from December 2012 to March 2013 (full methods have been described previously)( 21 ).
Breast-milk and interview data collection and analysis
In August 2014, we collected information on socio-economic demographics, household food security using the Household Food Insecurity Access Scale (HFIAS)( 22 ) and fish consumption (previous 3 d) with questionnaires administered to the breast-feeding mother in Dholuo, the local language. Maternal and infant weight and length/height were also measured (Seca 803 electronic scale, Seca 213 mobile stadiometer and Seca 417 infantometer).
Fish intake was quantified using a piloted questionnaire that quantified all fish consumption in the preceding 3 d. Pilot results showed that capturing household fish consumption required an extended recall period beyond that of a standard 24 h recall, as fish consumption was not adequately captured in the shorter time frame( 23 ). Quantity of fish consumed was recalled by participants with the aid of household measures (e.g. locally used spoons, bowls)( 24 , 25 ). The quantity of fish within a spoonful, bowl, etc. was then repeatedly measured and averaged to standardize quantities consumed. Extensive ethnographic observation on Mfangano demonstrated that dagaa and cichilds are consumed whole, so plate waste was not a concern; for Nile perch and tilapia, flesh, head and small bones are consumed, so plate waste was assumed to be negligible. Dagaa and cichlids are typically consumed dried, whereas tilapia and Nile perch are typically consumed fresh or fried for preservation and later boiled. To provide comparable measures of fish consumption, Nile perch and tilapia consumption was adjusted to remove moisture content using previously recorded moisture content values( 26 – 29 ).
Breast-milk spots were collected and dried on cards treated with OxyStop® antioxidants to ensure stability. Although fat content varies across fore and hind milk, fatty acid composition is relatively consistent( 30 ) and we collected foremilk in all samples and standardized to time of day, where participant schedules allowed. Eighty per cent of samples were collected from 12.00 to 17.00 hours, and 20 % from 09.30 to 12.00 hours. Samples were shipped from Kenya to the USA for analysis of breast-milk spots by OmegaQuant Analytics in Sioux Falls, SD (full procedures are described elsewhere( 31 )). An aliquot of fatty acid methyl esters was analysed with GC.
Fish sample data collection and analysis
Lake Victoria has two key commercial species: Nile perch (Lates niloticus) and dagaa (Rastrineobola argentea). Nile perch, an international export, was introduced by the British in the 1960s( 32 ) and drove more than 300 native cichlid species (Cichlidae) to extinction( 33 ). Local cichlid populations have rebounded slowly as a consequence of fishing pressure on Nile perch( 34 ). Similarly, as Nile perch catch declines, the small, sardine-like dagaa has grown in production and now represents the largest share of fish biomass caught in Lake Victoria. Introduced tilapia (Oreochromis niloticus) are also present in limited numbers due to high fishing pressure.
Samples of dagaa and mixed cichlid species (500 g) were harvested off Mfangano Island and sun-dried in accordance with local practices for approximately 8 h. Dried fish were kept at ambient temperature for 2 weeks during shipping. For each fish, multiple individuals (approximately twenty to thirty) were added and emulsified, then analysed by GLC using AOCS method Ce1b-89 (Covance Laboratories, Madison, WI, USA). Haplochromine cichlid species were analysed together because: (i) they are genetically similar, stemming from their adaptive radiation within Lake Victoria( 33 ), and occupy a similar position in the food web, meaning fatty acid content is similar across them; and (ii) they are harvested as juveniles and difficult to distinguish by species, meaning consumption of cichlids by households is not species-specific.
Statistical methods
We calculated descriptive statistics for all women and their child(ren) using the statistical software package STATA version 12. Lowess plots and bivariate regression were used to compare fatty acid concentrations in breast milk and fish consumption. We used multivariate regression to analyse associations between maternal consumption of specific fish species and breast-milk DHA, EPA, ALA, AA and LA while controlling for consumption of other fish and maternal BMI, maternal age, breast-feeding child’s age and exclusive breast-feeding status. While food insecurity is high in these communities, we did not elect to include it in our models because our previous work suggested that food insecurity and fish consumption (with fatty acid consumption defined here as a function of fish consumption) are highly intertwined, such that food insecurity is a function of fish consumption( 19 ). To address potential concerns about the role of socio-economic status in consumption of DHA, EPA, ALA, AA and LA, however, we provide models including food security in the online supplementary material, Supplemental Table 1. We identified DHA, EPA, ALA, AA and LA and maternal/child characteristics a priori.
Results
Women in our sample had a mean age of 27·3 (sd 5·6) years and a mean BMI of 22·8 (sd 3·2) kg/m2; 47 % (28/60) had some primary education and 35 % (21/60) had completed primary school. Women were breast-feeding children with a mean age of 11·7 (sd 7·4) months; 18 % (n 11) of children were breast-feeding exclusively. Thirty-one per cent (n 19) of children were stunted (8 % with length-for-age/height-for-age Z-score<−3; 23 % with length-for-age/height-for-age Z-score<−2), while 10 % of children were wasted (n 6; weight-for-height Z-score<−2). In the previous 3 d, 97 % (58/60) of women consumed fish, with a mean consumption of 178 (sd 111) g. Of the women consuming fish, 82 % of women consumed Nile perch and 77 % consumed dagaa in the previous 3 d. Cichlids and tilapia were consumed by 13% and 15 % of women, respectively. The mean food insecurity score was 8·5 (sd 4·7; range 0–22), with 98 % of households categorized as food insecure at some level, including severely (33 %), moderately (55 %) and mildly (10 %) food insecure (see online supplementary material, Supplemental Table 2).
Mean breast-milk DHA content was 0·75 % of total fatty acids, compared with a global average of 0·32 %, determined from a multi-country meta-analysis( 5 ) (Table 1). Mean breast milk AA content was 0·44 % compared with a global average of 0·47 %( 5 ). Further, dagaa contained 13·2%, 6·1%, 3·9%, 3·1% and 2·7 % of total fatty acids as DHA, EPA, ALA, AA and LA, respectively. Cichlid species contained smaller amounts of beneficial fatty acids (Table 1).
Table 1.
Fatty acid composition of fish predominantly consumed by the study population (percentage of total fatty acids) and mean breast-milk fatty acid composition (percentage of total fatty acids) among breast-feeding mothers (n 60) around Lake Victoria, Kenya, August 2014
| Fish species | Breast milk | ||||||
|---|---|---|---|---|---|---|---|
| Fatty acid | Dagaa | Cichlids | Nile perch† | Tilapia† | Mean | 25th–75th percentile | Global average‡ |
| Polyunsaturated | 32·6 | 14·1 | 35·9 | 38·9 | |||
| 18:2n-6 (LA) | 2·7 | 6·9 | – | – | 12·71 | 10·88–14·03 | |
| 18:3n-6 | 0·3 | 0·1 | 1·2 | 1·3 | 0·084 | 0·054–0·10 | |
| 20:3n-6 | 0·4 | 0·2 | – | – | 0·36 | 0·26–0·45 | |
| 20:4n-6 (AA) | 3·9 | 0·9 | 5·3 | 3·5 | 0·44 | 0·36–0·49 | 0·47 |
| 22:4n-6 | – | – | – | – | 0·091 | 0·07–0·10 | |
| 18:3n-3 (ALA) | 3·1 | 0·7 | 1·9 | 2·3 | 0·54 | 0·41–0·65 | |
| 20:5n-3 (EPA) | 6·1 | 0·8 | 4·5 | 3·7 | 0·16 | 0·11–0·21 | |
| 22:5n-3 (DPA) | 2·3 | 1·3 | 2 | 1·3 | 0·28 | 0·20–0·33 | |
| 22:6n-3 (DHA) | 13·2 | 3·2 | 15·9 | 13·7 | 0·75 | 0·54–0·89 | 0·32 |
| Monounsaturated | 23·9 | 30·7 | 26·5 | 18·7 | |||
| 16:1n-7 | 10·2 | 2·9 | 5·6 | 2·1 | 1·88 | 1·26–2·37 | |
| 18:1 | – | – | – | – | 30·42 | 27·96–33·76 | |
| 20:1 | 0·3 | 0·2 | – | – | 0·25 | 0·21–0·28 | |
| 24:1 | – | – | – | – | 0·072 | 0·047–0·081 | |
| Saturated | 41·7 | 54·1 | 33·1 | 39·6 | |||
| 10:0 | – | – | – | – | 0·67 | 0·44–0·85 | |
| 12:0 | – | – | – | – | 5·82 | 4·36–7·33 | |
| 14:0 | 3·7 | 1·4 | 1·4 | 0·8 | 11·07 | 7·63–13·16 | |
| 15:0 | 0·7 | 0·3 | 0·8 | 1·2 | |||
| 16:0 | 25·8 | 45·5 | 20·4 | 25·6 | 29·02 | 26·59–30·85 | |
| 17:0 | 2·0 | 0·5 | 0·6 | 0·9 | |||
| 18:0 | 9·1 | 6 | 9·9 | 11·1 | 4·16 | 3·75–4·50 | |
| 20:0 | 0·3 | 0·4 | – | – | 0·17 | 0·14–0·19 | |
| 22:0 | – | – | – | – | 0·088 | 0·066–0·10 | |
| 24:0 | 0·5 | 0·2 | – | – | 0·10 | 0·065–0·12 | |
Linear plots described breast-milk fatty acid concentrations and total fish consumption, as depicted in Fig. 1, although associations were not significant in bivariate regression models. However, multivariate regression models controlling for consumption of other fish and maternal and child covariates provided evidence that the species of fish consumed influenced breast-milk fatty acid concentrations (Table 2). Cichlid consumption was significantly associated with breast-milk LA, AA and ALA concentrations while controlling for maternal and child characteristics. Dagaa consumption was associated with breast-milk EPA concentrations, and tilapia with breast-milk ALA while controlling for maternal and child characteristics. Furthermore, results pooling small, native species (dagaa, cichlids) and large, non-native species (tilapia, Nile perch) showed similar patterns and are provided in the online supplementary material, Supplemental Table 3.
Fig. 1.
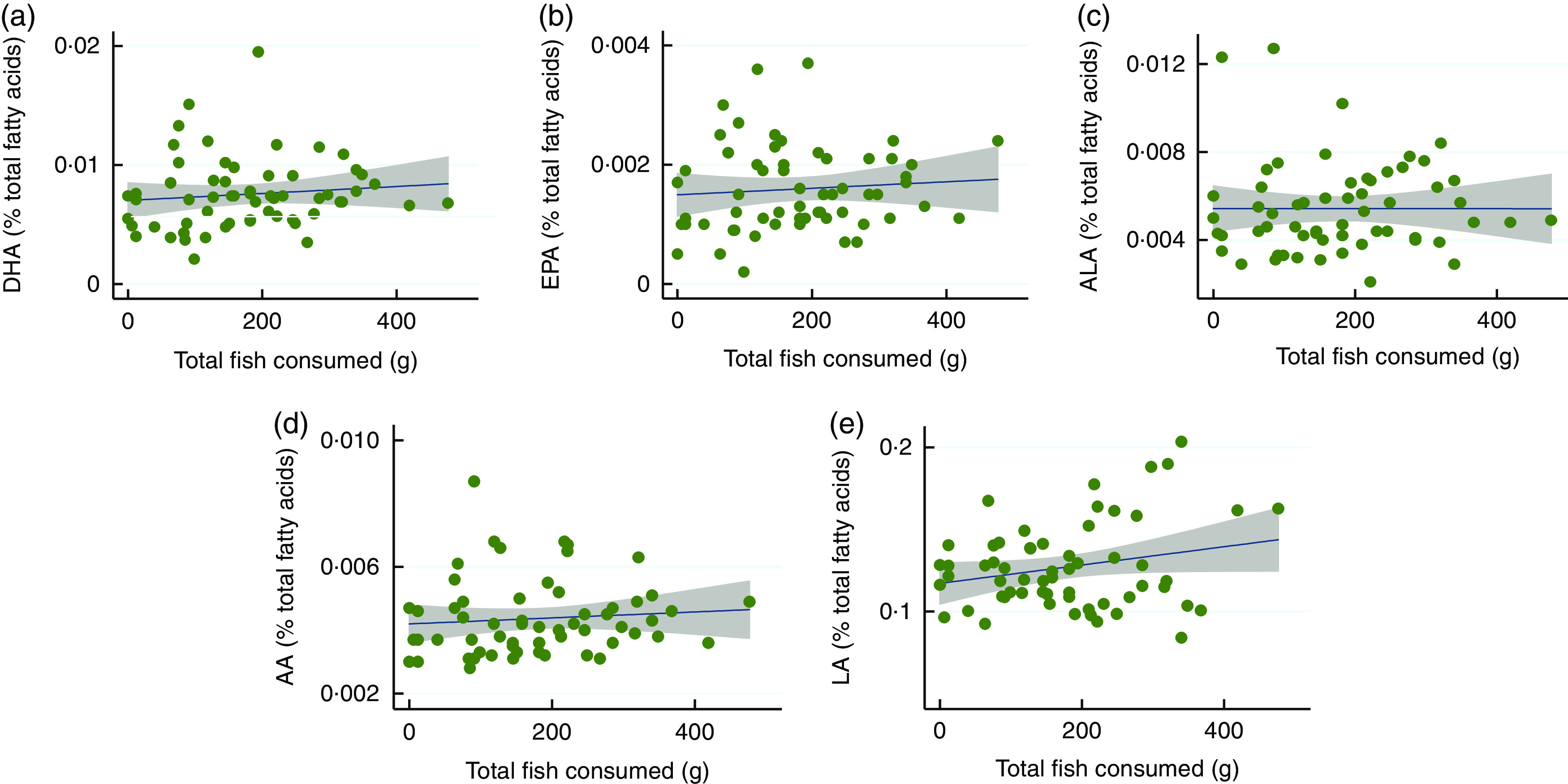
Linear regression plots depicting the association between total fish consumption over the previous 3 d and breast-milk long-chain PUFA (LCPUFA) concentrations among breast-feeding mothers (n 60) around Lake Victoria, Kenya, August 2014: (a) DHA, (b) EPA, (c) α-linolenic acid (ALA), (d) linoleic acid (LA) and (e) arachidonic acid (AA); 95 % CI are shaded. Results of multiple bivariate regression models of total fish consumption over the previous 3 d, normalized to three 85 g servings (predictor variable), and breast-milk levels of each LCPUFA (outcome variables) are: (a) coefficient=0·074 (95 % CI −0·11, 0·25), P=0·41; (b) coefficient=0·014 (95 % CI −0·029, 0·057), P=0·52; (c) coefficient=0·00 (95 % CI −0·12, 0·12), P=0·99; (d) coefficient=0·024 (95 % CI −0·048, 0·096), P=0·51; (e) coefficient=1·4 (95 % CI −0·11, 2·9), P=0·069
Table 2.
Associations between fish consumption (85 g of indicated fish species) and breast-milk levels of long-chain PUFA, while controlling for maternal and child characteristics, among breast-feeding mothers (n 60) around Lake Victoria, Kenya, August 2014
| Fish consumption | Maternal/child characteristics | Full models | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Predictor | Coef. | se | 95 % CI | Coef. | se | 95 % CI | Coef. | se | 95 % CI |
| DHA | Cichlids | 0·0033 | 0·0042 | −0·0052, 0·0118 | 0·0042 | 0·0042 | −0·0042, 0·0125 | |||
| Dagaa | 0·0022 | 0·0019 | −0·0016, 0·0060 | 0·0021 | 0·0019 | −0·0016, 0·0059 | ||||
| Tilapia | 0·0002 | 0·0036 | −0·0071, 0·0074 | −0·0007 | 0·0036 | −0·0079, 0·0065 | ||||
| Nile perch | 0·0008 | 0·0015 | −0·0022, 0·0037 | 0·0011 | 0·0015 | −0·0018, 0·0041 | ||||
| BMI | 0·0000 | 0·0001 | −0·0002, 0·0002 | 0·0000 | 0·0001 | −0·0003, 0·0002 | ||||
| Excluding EBF | −0·0026** | 0·0011 | −0·0049, −0·0003 | −0·0028** | 0·0012 | −0·0052, −0·0005 | ||||
| Mother’s age | 0·0000 | 0·0001 | −0·0002, 0·0001 | 0·0000 | 0·0001 | −0·0002, 0·0001 | ||||
| Child’s age | 0·0001 | 0·0001 | −0·0001, 0·0002 | 0·0001 | 0·0001 | −0·0000, 0·0002 | ||||
| EPA | Cichlids | 0·0010 | 0·0010 | −0·0010, 0·0030 | 0·0012 | 0·0010 | −0·0008, 0·0032 | |||
| Dagaa | 0·0008* | 0·0004 | −0·0001, 0·0017 | 0·0008* | 0·0004 | −0·0001, 0·0017 | ||||
| Tilapia | −0·0003 | 0·0008 | −0·0020, 0·0014 | −0·0005 | 0·0008 | −0·0022, 0·0012 | ||||
| Nile perch | 0·0001 | 0·0003 | −0·0006, 0·0008 | 0·0002 | 0·0004 | −0·0005, 0·0009 | ||||
| BMI | 0·0000 | 0·0000 | −0·0001, 0·0000 | 0·0000 | 0·0000 | −0·0001, 0·0000 | ||||
| Excluding EBF | −0·0005 | 0·0003 | −0·0010, 0·0001, | −0·0005* | 0·0003 | −0·0011, 0·0000 | ||||
| Mother’s age | 0·0000 | 0·0000 | 0·0000, 0·0000 | 0·0000 | 0·0000 | 0·0000, 0·0000 | ||||
| Child’s age | 0·0000 | 0·0000 | 0·0000, 0·0000 | 0·0000 | 0·0000 | 0·0000, 0·0001 | ||||
| ALA | Cichlids | 0·0056** | 0·0027 | 0·0001, 0·0111 | 0·0061** | 0·0026 | 0·0010, 0·0113 | |||
| Dagaa | −0·0014 | 0·0012 | −0·0038, 0·0011 | −0·0006 | 0·0012 | −0·0029, 0·0017 | ||||
| Tilapia | 0·0043* | 0·0023 | −0·0003, 0·0090 | 0·0042* | 0·0022 | −0·0002, 0·0086 | ||||
| Nile perch | −0·0004 | 0·0009 | −0·0022, 0·0015 | 0·0003 | 0·0009 | −0·0015, 0·0021 | ||||
| BMI | −0·0001* | 0·0001 | −0·0003, 0·0000 | −0·0002** | 0·0001 | −0·0003, 0·0000 | ||||
| Excluding EBF | −0·0003 | 0·0007 | −0·0018, 0·0012 | −0·0004 | 0·0007 | −0·0018, 0·0010 | ||||
| Mother’s age | 0·0001* | 0·0000 | 0·0000, 0·0002 | 0·0001** | 0·0000 | 0·0000, 0·0002 | ||||
| Child’s age | 0·0001** | 0·0000 | 0·0000, 0·0002 | 0·0001 | 0·0000 | 0·0000, 0·0002 | ||||
| AA | Cichlids | 0·0029* | 0·0017 | −0·0004, 0·0063 | 0·0032* | 0·0017 | −0·0002, 0·0066 | |||
| Dagaa | −0·0004 | 0·0007 | −0·0019, 0·0011 | −0·0004 | 0·0008 | −0·0019, 0·0012 | ||||
| Tilapia | −0·0004 | 0·0014 | −0·0033, 0·0024 | −0·0007 | 0·0015 | −0·0036, 0·0023 | ||||
| Nile perch | 0·0003 | 0·0006 | −0·0008, 0·0015 | 0·0005 | 0·0006 | −0·0007, 0·0017 | ||||
| BMI | 0·0000 | 0·0001 | −0·0001, 0·0001 | 0·0000 | 0·0001 | −0·0001, 0·0001 | ||||
| Excluding EBF | −0·0005 | 0·0005 | −0·0015, 0·0004 | −0·0007 | 0·0005 | −0·0016, 0·0003 | ||||
| Mother’s age | 0·0000 | 0·0000 | −0·0001, 0·0001 | 0·0000 | 0·0000 | −0·0001, 0·0001 | ||||
| Child’s age | 0·0000 | 0·0000 | 0·0000, 0·0001 | 0·0000 | 0·0000 | 0·0000, 0·0001 | ||||
| LA | Cichlids | 0·0940*** | 0·0344 | 0·0251, 0·1630 | 0·0940** | 0·0359 | 0·0220, 0·1661 | |||
| Dagaa | 0·0021 | 0·0152 | −0·0286, 0·0327 | 0·0017 | 0·0162 | −0·0308, 0·0342 | ||||
| Tilapia | 0·0386 | 0·0295 | −0·0204, 0·0976 | 0·0391 | 0·0309 | −0·0230, 0·1011 | ||||
| Nile perch | 0·0166 | 0·0119 | −0·0073, 0·0405 | 0·0169 | 0·0128 | −0·0087, 0·0425 | ||||
| BMI | 0·0004 | 0·0011 | −0·0018, 0·0027 | −0·0002 | 0·0011 | −0·0024, 0·0020 | ||||
| Excluding EBF | 0·0039 | 0·0103 | −0·0168, 0·0246 | 0·0012 | 0·0099 | −0·0187, 0·0211 | ||||
| Mother’s age | −0·0001 | 0·0007 | −0·0014, 0·0012 | −0·0001 | 0·0006 | −0·0014, 0·0011 | ||||
| Child’s age | −0·0002 | 0·0005 | −0·0013, 0·0009 | 0·0000 | 0·0005 | −0·0011, 0·0011 | ||||
Coef., coefficient; ALA, α-linolenic acid; AA, arachidonic acid; LA, linoleic acid; EBF, exclusive breast-feeding.
The P<0·01 level of significance corrects for multiple comparisons.
*P<0·1, **P<0·05, ***P<0·01.
Discussion
We found high levels of beneficial breast-milk LCPUFA and essential n-3 and n-6 fatty acids among women living around Lake Victoria, Kenya. Women in the sample consumed approximately 2 servings of fish in the previous 3 d, on average. Breast-milk DHA levels well exceeded global and regional averages( 5 , 35 ), with fifty-nine of sixty samples having DHA concentration above the 0·32 % global average. High DHA levels were comparable to levels in lakeside regions of Malawi( 13 ). EPA levels were also strikingly high, while LA and ALA levels were lower than global averages but comparable regionally( 35 ). AA levels were lower than expected given the quantities of fish consumed, although similar to global averages( 5 , 35 ).
Despite the high aggregate consumption of fish in this population, the relative composition of diets from different fish species uniquely contributed to the fatty acid profile of women’s breast milk. We observed a positive association between high consumption of dagaa and EPA concentrations. Further, high cichlid consumption was positively associated with LA, AA and ALA concentrations, and high tilapia consumption was positively associated with ALA consumption. These findings suggest differences in the composition of fish in diets. Even within a region consuming high quantities of fish, the fish species consumed may have bearing on the fatty acid composition of breast milk. The unexpected lack of an association between fish intake and breast-milk DHA concentrations, as well as between total fish intake and breast-milk fatty acid concentrations, could be due to the relatively high levels of fish consumption and high levels of breast-milk LCPUFA across our sample, which could have masked this relationship. Noise around dietary intake estimates, a small sample size, or factors around DHA metabolism and the timing of sample collection may also have been contributing factors.
Local populations are often relegated to consumption of specific fish species, typically to those with low economic value( 28 ). Yet ensuring continued access to nutritionally rich and diverse fish species is likely to be particularly important for maternal and child nutrition. Analysis of native fish species (cichlids and dagaa) directly addresses the natural resources that underpin access to high-quality diets and shape fatty acid concentrations. Dagaa and cichlids provide a good source of DHA, EPA and AA. An 85 g serving of dagaa contains 847 mg DHA, 390 mg EPA and 295 mg AA, and of cichlids contains 536 mg DHA, 129 mg EPA and 153 mg AA. A single serving of dagaa exceeds the recommended intake of 300 mg DHA+EPA daily and is comparable to usipe, a similar species native to Lake Malawi( 13 ). Analysis of nutrient composition of important fish species in Bangladesh further underscores the nutritional value of small indigenous fish species( 36 ).
Our data provide evidence of the nutritional and health importance of tropical freshwater fish in general, and particularly of the native fish species in Lake Victoria. While such species have been underappreciated within a fisheries discourse focused on regional economic potential, these fish remain critical to local diets. Dagaa is presently accessible, affordable and a mainstay in diets, while cichlid catches remain limited as a consequence of the Nile perch introduction. Although dagaa is currently less threatened by overfishing than the larger Nile perch, ensuring the availability of dagaa for local consumption requires continued attention to fisheries governance and careful planning for any expansion of aquaculture, which will likely rely on these low-value fish for fishmeal and focus on tilapia production. The nutritional importance of local species for mothers and their children suggests that fishery management ought to consider not only the economic benefits of exported species like Nile perch, but also the nutritional contributions of all fish populations. Further, despite documentation of the highest LCPUFA levels in coldwater marine fish species, our results demonstrate the importance of tropical freshwater fish resources for the LCPUFA supply.
Our cross-sectional study design prevents causal interpretations. Although our sample size is small and post hoc power calculations are limited( 37 ), such calculations suggest our sample was sufficient to assess effects of fish consumed on fatty acid concentrations. Our analysis of fish intake was limited by the estimation of quantitative intake from recall data, which is subject to recall bias, social desirability bias and trends in over/underestimation. Further, the extrapolation of fish consumption to fatty acid consumption relies on estimation, fatty acid composition analyses replication was limited, and we did not include other potential dietary sources of LCPUFA. We cannot draw conclusions about outcomes for infants as we did not measure children’s fatty acid levels. Finally, we compared our results with previously published information and differences may reflect both biological and analytical differences.
Conclusions
Kenyan women living on the shores of Lake Victoria consumed high quantities of fish and had high levels of breast-milk LCPUFA, particularly DHA and AA. The relative fatty acid composition of these women’s breast milk was shaped by the specific fish species they consumed. Safeguarding access to relatively available and affordable local fish, particularly dagaa and haplochromine cichlids, may be instrumental in shaping breast-milk LCPUFA concentrations and should remain a priority in public health and fisheries management strategies.
Acknowledgements
Acknowledgements: The authors appreciate the support for this research from Organic Health Response and the Mfangano Community. They gratefully acknowledge the research staff who contributed to this study, especially Jackline Luware, Mary Janet Achieng, Vallary Migawi, Beatrice Awuor, Maureen Okeyo and Janet Awino. They also thank Charles Salmen, Matthew Hickey, and two anonymous reviewers for feedback on earlier versions of this manuscript. Financial support: This work was supported by a National Science Foundation Doctoral Dissertation Research Improvement grant (K.J.F.) and received partial support from a National Science Foundation Geosciences grant (grant number 115057). The funders had no role in the design, analysis or writing of this article. Conflict of interest: The authors declare no conflicts of interest. Authorship: K.J.F., E.M.M. and L.C.H.F. developed the study. E.B. provided guidance on data collection and ethical considerations. K.J.F. and E.M.M. carried out the data collection. K.J.F. performed the statistical analysis, with input from co-authors. K.J.F. and E.M.M. wrote the manuscript with feedback and detailed review from all co-authors. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee of the Kenya Medical Research Institute and the Committee for Protection of Human Subjects at the University of California, Berkeley. Written informed consent was obtained from all subjects.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980017003147.
click here to view supplementary material
References
- 1. Koletzko B, Lien E, Agostoni C et al. (2008) The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med 36, 5–14. [DOI] [PubMed] [Google Scholar]
- 2. Brown JE, Isaacs J, Krinke B et al. (2013) Nutrition Through the Life Cycle, 5th ed. Stamford, CT: Cengage Learning. [Google Scholar]
- 3. Lauritzen L & Carlson SE (2011) Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status. Matern Child Nutr 7, 41–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koletzko B, Cetin I, Brenna JT et al. (2007) Dietary fat intakes for pregnant and lactating women. Br J Nutr 98, 873–877. [DOI] [PubMed] [Google Scholar]
- 5. Brenna JT, Varamini B, Jensen RG et al. (2007) Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr 85, 1457–1464. [DOI] [PubMed] [Google Scholar]
- 6. Lauritzen L, Jorgensen MH, Hansen HS et al. (2002) Fluctuations in human milk long-chain PUFA levels in relation to dietary fish intake. Lipids 37, 237–244. [DOI] [PubMed] [Google Scholar]
- 7. Lauritzen L, Jorgensen MH & Michaelsen KF (2000) Dietary fish and the docosahexaenoic acid (DHA) content of human milk. In Short and Long Term Effects of Breast Feeding on Child Health. Advances in Experimental Medicine and Biology Series vol. 478, pp. 403–404 [B Koletzko, KF Michaelsen and O Hernell, editors]. New York: Kluwer Academic/Plenum. [DOI] [PubMed] [Google Scholar]
- 8. Quinn EA & Kuzawa CW (2012) A dose–response relationship between fish consumption and human milk DHA content among Filipino women in Cebu City, Philippines. Acta Paediatr 101, E439–E445. [DOI] [PubMed] [Google Scholar]
- 9. Tiangson CLE, Gavino VC, Gavino G et al. (2003) Docosahexaenoic acid level of the breast milk of some Filipino women. Int J Food Sci Nutr 54, 379–386. [DOI] [PubMed] [Google Scholar]
- 10. Quinn EA, Largado F, Power M et al. (2012) Predictors of breast milk macronutrient composition in Filipino mothers. Am J Hum Biol 24, 533–540. [DOI] [PubMed] [Google Scholar]
- 11. Olang B, Hajifaraji M, Ali MA et al. (2012) Docosahexaenoic acid in breast milk reflects maternal fish intake in Iranian mothers. Food Nutr Sci 3, 441–446. [Google Scholar]
- 12. Lee PS, Wickramasinghe VP, Lamabadusuriya SP et al. (2013) Breast milk DHA levels in Sri Lankan mothers vary significantly in three locations that have different access to dietary fish. Ceylon Med J 58, 51–55. [DOI] [PubMed] [Google Scholar]
- 13. Yakes Jimenez E, Mangani C, Ashorn R et al. (2015) Breast milk from women living near Lake Malawi is high in docosahexaenoic acid and arachidonic acid. Prostaglandins Leukot Essent Fatty Acids 95, 71–78. [DOI] [PubMed] [Google Scholar]
- 14. Hibbeln JR, Davis JM, Steer C et al. (2007) Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet 369, 578–585. [DOI] [PubMed] [Google Scholar]
- 15. Oken E, Osterdal ML, Gillman MW et al. (2008) Associations of maternal fish intake during pregnancy and breastfeeding duration with attainment of developmental milestones in early childhood: a study from the Danish National Birth Cohort. Am J Clin Nutr 88, 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mohanty BP, Ganguly S, Mahanty A et al. (2016) DHA and EPA content and fatty acid profile of 39 food fishes from India. BioMed Res Int 2016, 4027437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Food and Agriculture Organization of the United Nations (2012) The State of World Fisheries and Aquaculture. Rome: FAO. [Google Scholar]
- 18. Kawarazuka N & Bene C (2010) Linking small-scale fisheries and aquaculture to household nutritional security: an overview. Food Secur 2, 343–357. [Google Scholar]
- 19. Fiorella KJ, Hickey MD, Salmen CR et al. (2014) Fishing for food? Analyzing links between fishing livelihoods and food security around Lake Victoria, Kenya. Food Secur 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Briend A, Dewey KG & Reinhart GA (2011) Fatty acid status in early life in low-income countries – overview of the situation, policy and research priorities. Matern Child Nutr 7, 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fiorella KJ, Camlin CS, Salmen CR et al. (2015) Transactional fish-for-sex relationships amid declining fish access in Kenya. World Dev 74, 323–332. [Google Scholar]
- 22. Coates J, Swindale A & Bilinsky P (2007) Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide. Washington, DC: Food and Nutrition Technical Assistance III Project (FANTA), FHI 360. [Google Scholar]
- 23. Wentland E & Smith K (1993) Survey Responses: An Evaluation of Their Validity. New York: Academic Press. [Google Scholar]
- 24. Cypel YS, Guenther PM & Petot GJ (1997) Validity of portion-size measurement aids: a review. J Am Diet Assoc 97, 289–292. [DOI] [PubMed] [Google Scholar]
- 25. Loevinsohn M (2015) The 2001–03 famine and the dynamics of HIV in Malawi: a natural experiment. PLoS One 10, e0135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okeyo GO, Lokuruka MNI & Matofari JW (2009) Nutritional composition and shelflife of the Lake Victoria Nile perch (Lates niloticus) stored in ice. Afr J Food Agr Nutr Dev 9, 901–919. [Google Scholar]
- 27. Kabahenda M, Mbabazi J, Kwetegyeka J et al. (2012) Nutrient alterations in Nile perch (Lates niloticus) skins owing to various processing and cooking techniques. Int J Environ Stud 69, 111–119. [Google Scholar]
- 28. Kabahenda MK, Amega R, Okalany E et al. (2011) Protein and micronutrient composition of low-value fish products commonly marketed in the Lake Victoria region. World J Agric Sci 7, 521–526. [Google Scholar]
- 29. Puwastien P, Judprasong K, Kettwan E et al. (1999) Proximate composition of raw and cooked Thai freshwater and marine fish. J Food Compost Anal 12, 9–16. [Google Scholar]
- 30. Koletzko B, Mrotzek M & Bremer H (1986) Fat content and cis- and trans-isomeric fatty acids in human fore- and hindmilk. In Human Lactation 2. Maternal and Environmental Factors, pp. 589–601 [M Hamosh and AS Goldman, editors]. New York: Plenum Press. [Google Scholar]
- 31. Jackson KH, Polreis J, Sanborn L et al. (2016) Analysis of breast milk fatty acid composition using dried milk samples. Int Breastfeed J 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pringle RM (2005) The origins of the Nile perch in Lake Victoria. BioScience 55, 780–787. [Google Scholar]
- 33. Witte F, Goldschmidt T, Wanink J et al. (1992) The destruction of an endemic species flock – quantitative data on the decline of the haplochromine cichlids of Lake Victoria. Environ Biol Fishes 34, 1–28. [Google Scholar]
- 34. Njiru M, Mkumbo OC & van der Knaap M (2010) Some possible factors leading to decline in fish species in Lake Victoria. Aquat Ecosyst Health Manag 13, 3–10. [Google Scholar]
- 35. Li S-Y, Dong Z-L, Wong W-SV et al. (2015) Long-chain polyunsaturated fatty acid concentrations in breast milk from Chinese mothers: comparison with other regions. Int J Child Health Nutr 4, 230–239. [Google Scholar]
- 36. Bogard JR, Thilsted SH, Marks GC et al. (2015) Nutrient composition of important fish species in Bangladesh and potential contribution to recommended nutrient intakes. J Food Compost Anal 42, 120–133. [Google Scholar]
- 37. Bacchetti P (2010) Current sample size conventions: flaws, harms, and alternatives. BMC Med 8, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Masa J, Ogwok P, Muyonga JH et al. (2011) Fatty acid composition of muscle, liver, and adipose tissue of freshwater fish from Lake Victoria, Uganda. J Aquat Food Prod Technol Health Care 20, 64–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980017003147.
click here to view supplementary material


