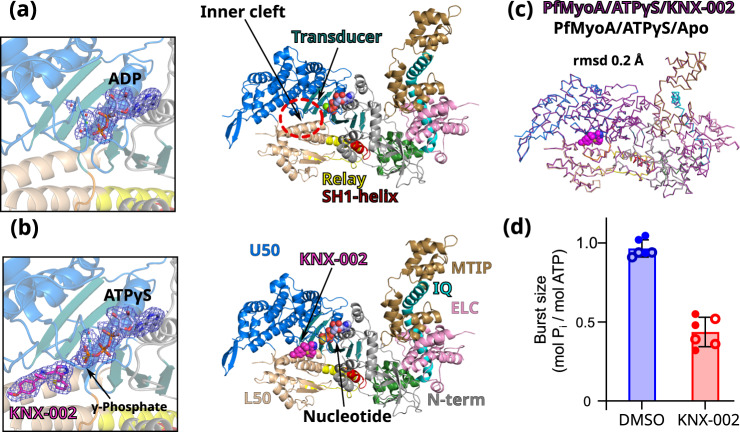
Fig. 2. KNX-002 targets the post-rigor (PR) state.
a Structure of PfMyoA in the apo condition (PfMyoA/ATPγS/Apo). Only ADP is found in the 2Fo-Fc electron density map contoured at 1.0 σ (on the left). ATPyS was thus hydrolyzed by myosin, in contrast to the same experiment performed in the presence of KNX-002. b Structure of PfMyoA complexed with KNX-002 and MgATPyS (PfMyoA/ATPγS/KNX-002). The compound, MgATPyS, the water molecules and the Mg2+ ion can be clearly identified in the 2Fo-Fc electron density map contoured at 1.0 σ (on the left). The gamma-phosphate of ATPyS is present in the density. U50, Upper 50 kDa subdomain; L50, Lower 50 kDa subdomain. c The compound does not induce major structural rearrangements upon binding. PfMyoA/ATPγS/KNX-002 and PfMyoA/ATPγS/Apo are both in a PR state and superimpose quite well with a rmsd of 0.2 Å using the Cα atoms. Zoom on the regions with maximum differences between the two structures show local displacements of side chains (see Supplementary Fig. 3a). d Manual quenching experiments show a decreased phosphate burst from 0.97 ± 0.05 mol Pi/mol ATP in the absence of compound to 0.44 ± 0.08 mol Pi/mol ATP in the presence of 100 µM KNX-002 (p < 0.0001, two-tailed t-test with Welch’s correction). Data represent two experiments each performed in triplicate with independent protein preparations (open and filled circles). Data bars are mean ± SD. Source data are provided as a Source Data file.