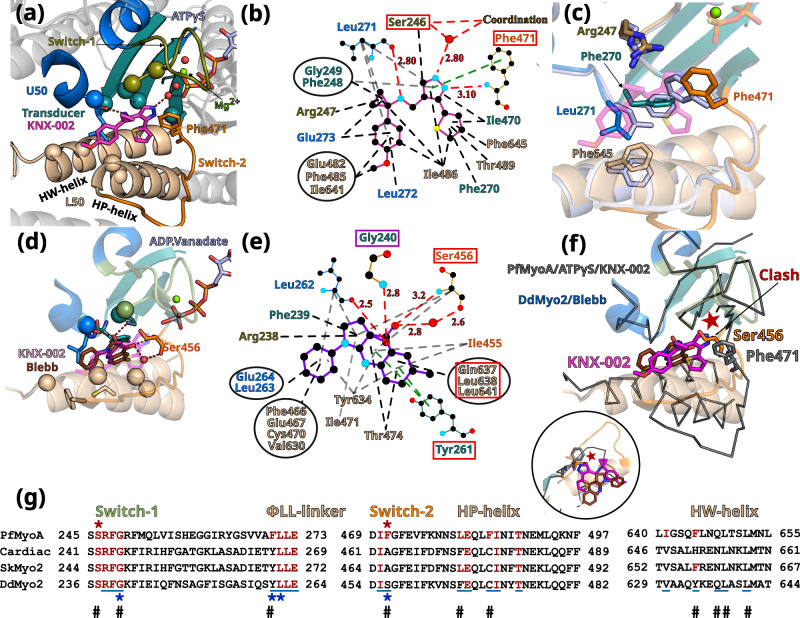
Fig. 3. The inner pocket in which KNX-002 binds greatly differs from that of Blebbistatin (Blebb).
a KNX-002 binding pocket. Regions involved in binding are: Switch-2 (orange); Transducer (deep teal cyan); U50 (marine blue); L50 (wheat). Residues are displayed as spheres when involved in apolar interactions; as sticks when involved in electrostatic or π-stacking bonds. b Schematic representation of the binding pocket of KNX-002. Each type of interaction is represented differently (Polar interactions, red dashed lines; π-stacking, green; apolar, black). Squares indicate residues involved in different types of bonds for KNX-002 and Blebbistin (shown in e). c Superimposition of PfMyoA/ATPγS/KNX-002 (colored by subdomains) and PfMyoA/ATPγS/Apo (gray-blue), both in the post-rigor state. Residues with different conformation (sticks), indicate how adjustments are required to bind KNX-002. d Blebb binding pocket in Dictyostelium discoideum myosin 2 (DdMyo2, PDB code 1YV328) with the U50 subdomain orientation as in 3a for PfMyoA. KNX-002 (pale purple) does not fit in this pocket as the pre-powerstroke conformation of Switch-2 clashes with the KNX-002 position. Supplementary Table 2 and Supplementary Movie 3 further illustrate how the KNX-002 and Blebb binding sites differ. e Schematic representation of interactions around Blebb. Residues that differ from PfMyoA but bind Blebb are in a red box; conserved residue involved in different bond types (purple box). f KNX-002 and Blebb target different pockets. DdMyo2/Blebb (cartoon, colored by subdomain) and PfMyoA/ATPγS/KNX-002 (ribbon, colored in black) are superimposed on the U50 subdomain (residues 182-463 and 604-631). KNX-002 and Blebb binding pockets strongly differ in the conformation of Switch-2 and in the orientation of HP- and HW-helices. Another orientation is represented as a zoom in a circle. g Sequence alignment of PfMyoA, β-cardiac MYH7 (cardiac), skeletal muscle myosin 2 (SkMyo2) and DdMyo2 for analysis of the conservation of residues involved in KNX-002 and Blebb binding. Residues involved in KNX-002 binding are colored red in PfMyoA and in other myosins when conserved. Residues involved in Blebb binding are underlined (blue). Residues involved in electrostatic or stacking interactions with KNX-002 (red star), and those involved with Blebb (blue star) are indicated. Remarkable positions that distinguish KNX-002 and Blebb binding modes are marked with a #.