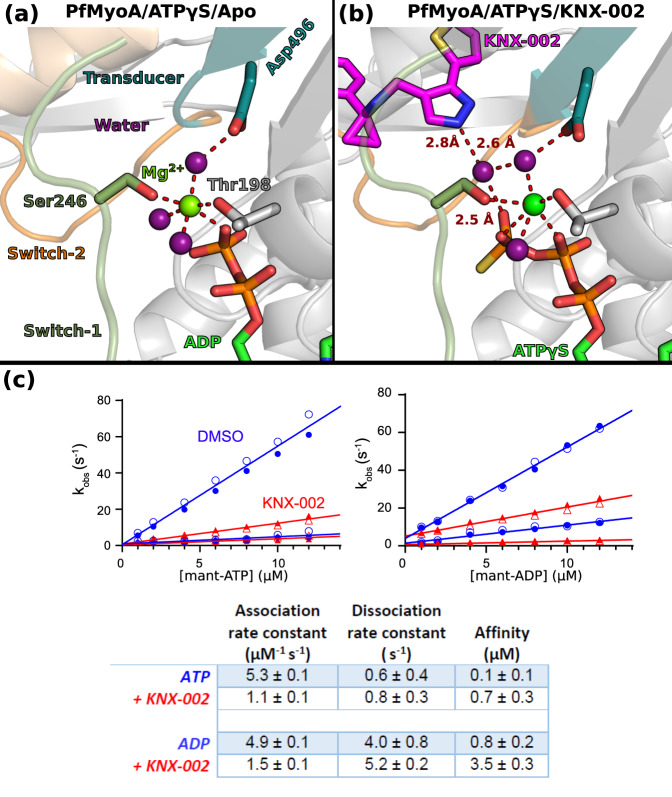
Fig. 4. Effects of KNX-002 on the active site.
The Mg2+ ion is hexa-coordinated in both the Apo (a) and in the KNX-002 bound (b) structures. a Although ATPγS was used for the crystallization in both structures, the nucleotide is hydrolyzed in the absence of KNX-002 and ADP is found in the active site (a). b When KNX-002 occupies its pocket, the compound stabilizes a water molecule that also binds the γ-Pi of ATP and a water molecule that coordinates the Mg2+ ion. Supplementary Fig. 7 indicates that this additional interaction does not change the hexa-coordination of the Mg2+ ion. c KNX-002 slows both mant-ATP and mant-ADP binding to PfMyoA. Rates in the absence (blue circles) or presence of 100 µM KNX-002 (red triangles) of the fast and slow phases from the biphasic transients are plotted. The association (slope) and dissociation (y-intercept) rate constants and resulting affinity are given in the Table. Data represent two experiments with independent protein preparations. Source data are provided as a Source Data file.