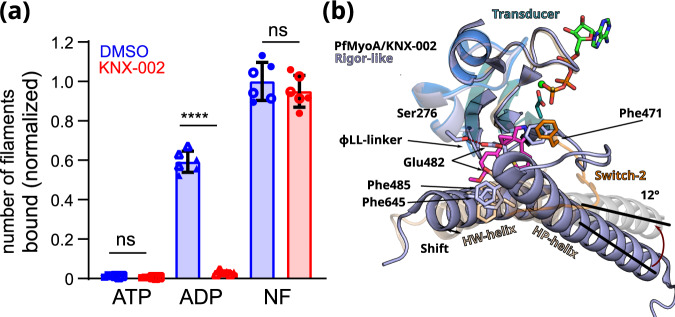
Fig. 5. PfMyoA.ADP binds actin weakly in the presence of KNX-002.
a KNX-002 weakens the affinity of actin for M.ADP but has no effect on binding in the presence of ATP or with nucleotide-free (NF)-PfMyoA. Number of actin filaments bound ± SD to surface immobilized PfMyoA (see Methods). See Supplementary Fig. 8 for examples of the raw data and Supplementary Fig. 9 for data with an expanded y-axis scale. The difference between ADP ± KNX-002 is significant (p < 0.0001) (one-sided ANOVA followed by Tukey’s post-hoc test), but there were no significant differences between ATP ± KNX-002 (p > 0.999), nor between NF ± KNX-002 (p = 0.64). Data represent two experiments each performed in triplicate with independent protein preparations (open and filled circles). Data bars are mean ± SD. Source data are provided as a Source Data file. b The KNX-002 binding pocket does not exist in the Rigor state. PfMyoA/ATPγS/KNX-002 (colored by subdomains as in Fig. 2b) and PfMyoA in the Rigor-like state (light blue) (PDB code 6I7D9) are superimposed on the U50 subdomain and show how a change in the conformation and the orientation of the L50 subdomain would close the inner cleft and would thus not be compatible with KNX-002 binding. Indeed, the L50HP-helix rotates by 12° and the L50HW-helix shifts, changing the position of F485 and F645, two residues involved in KNX-002 binding. The reorientation of Switch-2 also leads to a new position for the key F471 residue that is incompatible with KNX-002 docking into this site in the Rigor state.