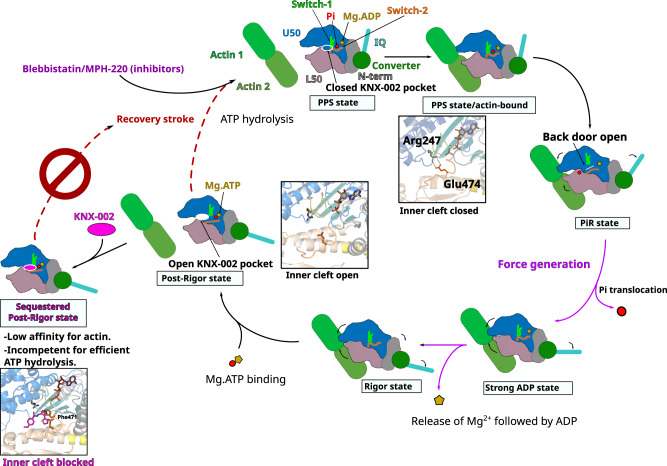
Fig. 6. Mechanism of the inhibition by compound KNX-002.
Schematic representation of the motor cycle of PfMyoA, where the state of nucleotide bound governs (i) the conformation of the motor and (ii) the affinity for the actin filament. In the post-rigor (PR) state, PfMyoA binds Mg.ATP and has low affinity for actin since the actin-binding cleft between U50 and L50 subdomains is open. After the recovery stroke that primes the lever arm, PfMyoA adopts the pre-powerstroke (PPS) state in which ATP is hydrolyzed. The weak association of the PPS to actin initiates a transition towards the Pi release state (PiR), the first force-producing state. This initiation of the powerstroke allows the opening of the Pi release tunnel allowing Pi translocation. After Pi release, a large swing of the lever arm (powerstroke) leads to a Strong ADP state in which the actin-binding cleft is closed, allowing stronger association with actin. Mg2+ and ADP are finally released from the Rigor state after a small swing of the lever arm and reorientation of the N-terminal subdomain. An ATP molecule can then bind, which leads to a fast transition towards the post-rigor state that detaches from actin. In contrast to the previously described Myo2 inhibitors, Blebbistatin and MPH-22056 which target PPS, KNX-002 targets PR, which prevents the recovery stroke and ATP hydrolysis. When KNX-002 intercalates between the U50 and the L50, it stabilizes a state of poor affinity for F-actin that is not compatible with efficient ATP hydrolysis.