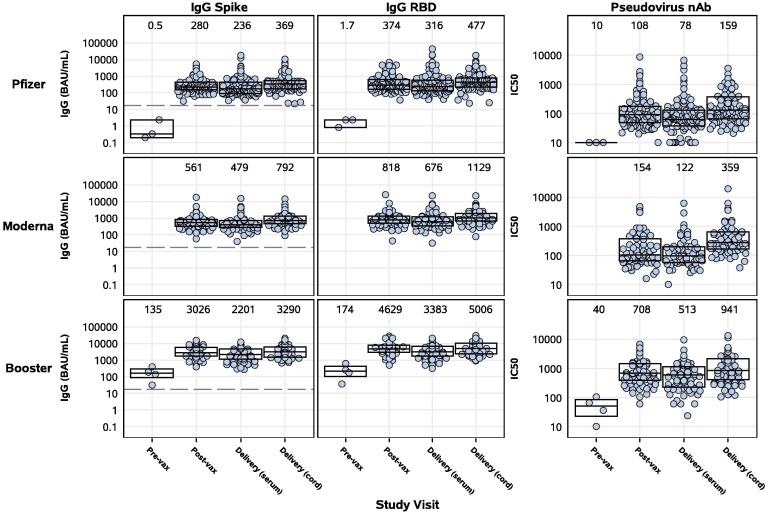
Figure 1.
SARS-CoV-2 binding IgG and pseudovirus nAb activity in maternal and cord blood sera by study group and study visit. Pregnant participants received a 2-dose series of an mRNA vaccine (top – Pfizer, middle – Moderna) or a booster mRNA vaccine (bottom panel). Sera derived from maternal blood collected pre- and post-vaccination and at delivery, and cord blood, were evaluated for binding IgG to full-length Spike (left panels) and RBD (middle panels), or pseudovirus nAb titers (IC50) (right panels). Binding IgG titers were bridged to international standards and reported as Binding Antibody Units (BAU/mL). Box plots represent median (horizontal line within the box) and interquartile range; GMT is displayed at the top of each panel and the dashed line is the cutoff for positivity (17 BAU/mL).