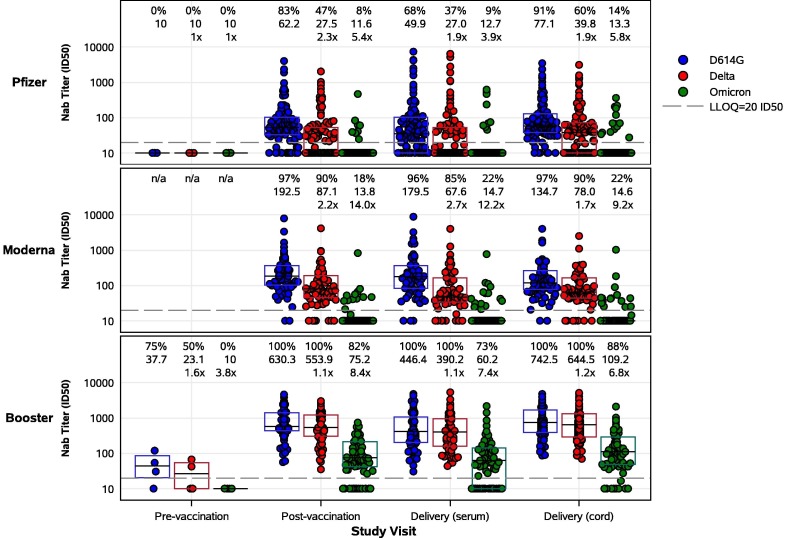
Figure 2.
SARS-CoV-2 live virus nAb activity of maternal and cord blood sera by study group and study visit. Pregnant participants received a 2-dose series of an mRNA vaccine (top – Pfizer, middle – Moderna) or a booster mRNA vaccine (bottom panel). Sera derived from maternal blood collected pre- and post-vaccination and at delivery, and cord blood, were evaluated for neutralization of D614G, Delta, and Omicron (BA.1) variants. Each point represents the GMT ID50 from two duplicates per specimen (within the same assay run). A value equivalent to half the lower limit of detection (LLOD = 20) was assigned to observations with no detectable response. A specimen was considered as having a positive response if at least one of the duplicates was above the LLOD. Box plots represent median (horizontal line within the box) and interquartile range. Response rate (% with responses > 20 ID50), GMT, and GMT fold reduction compared to D614G are displayed at the top of each panel.