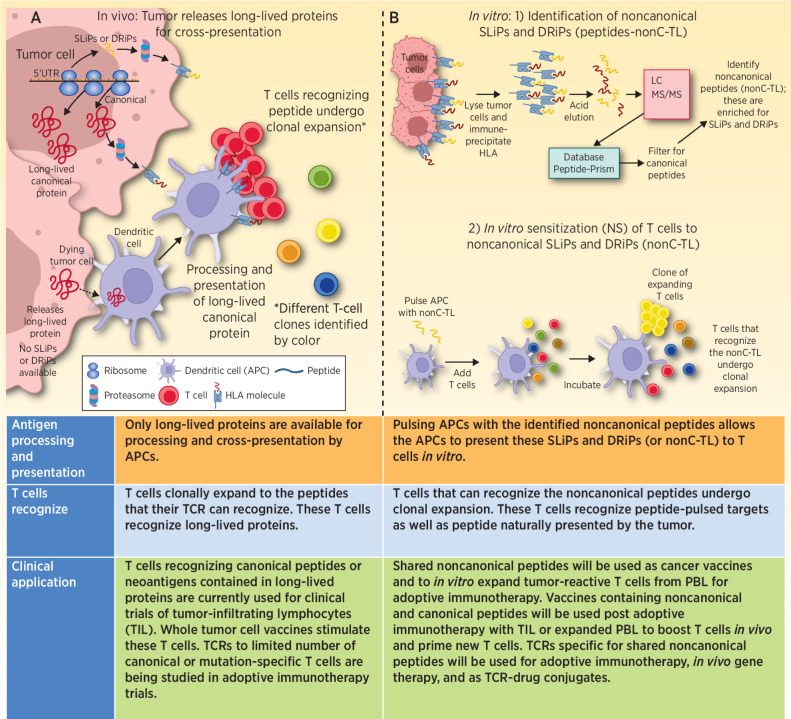
Figure 1.
A, Progressively growing tumors are transcribing and translating canonical proteins. At the same time, NCPs derived from uORFs, 5′UTRs, ncRNAs, EREs, pseudogenes, and out-of-frame transcripts may also be generated, some of which can contribute to malignant properties of the cancer. The majority of these NCPs are SLiPs and DRiPs that will be rapidly degraded, and presented by HLA, but because of their short-lived nature are unable to be picked up, processed, and presented by APCs. Thus, the patient is unable to generate an immune response to these short-lived noncanonical proteins. When tumor cells die, the proteins that are available to be picked up and processed are predominantly long-lived proteins. Peptides derived from these long-lived proteins are the dominant antigens that the patient makes a T-cell response against. B, 1) In vitro isolated tumor cells are lysed, antibody to HLA class I is incubated with the lysate, and HLA molecules are immunoprecipitated. The peptides are next eluted from the HLA molecule and run through LC/MS-MS to identify peptide sequences. These data are then queried using a database (Peptide-PRISM) that identifies the peptides that were bound to HLA. These data are then filtered for canonical proteins. The result is a list of NCPs presented by HLA. 2) These NCPs (nonC-TL) were used in IVS assays to prime and expand T cells able to recognize the NCPs. In some cases, the expanded T cells recognized the NCP pulsed onto APC. These also recognized naturally presented peptide on tumor cells and secreted IFNγ or upregulated 4–1BB expression. TCRs isolated from these expanded T cells also recognize the NCP.