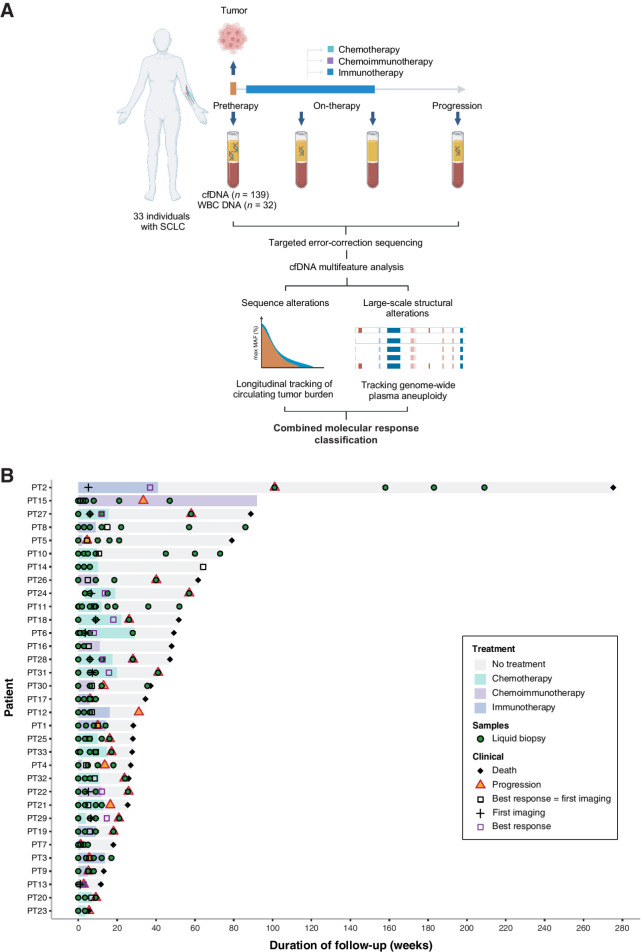
Figure 1.
Study methodology and cohort analyzed. Overall, 33 patients with SCLC who received systemic treatment with either chemotherapy or immunotherapy-containing regimens were analyzed for this study. A, High-depth targeted error-correction sequencing (TEC-seq) was performed on cfDNA extracted from serial plasma samples collected at baseline and longitudinally throughout the course of treatment, alongside matched WBC DNA. Sequence and structural alterations were directly detected in cfDNA for each patient at each time point analyzed and used to evaluate longitudinal changes in cfTL using a combined tiered approach. WES was further performed on pretreatment tumor and matched WBC DNA derived from buffy coat from a subset of 5 patients and used to evaluate the concordance between copy-number profiles computed from tumor next-generation sequence data and PA. Finally, molecular response classifications were assigned to each patient based on dynamic changes in cfTL and used to predict clinical outcomes. B, Swimmer plot showing disease course in each patient, treatment, and samples collected. The mean time to first blood sample collection after initiation of treatment for patients included in the study was 9 weeks (median 3 weeks). The mean time to first imaging assessment in this cohort was 8 weeks (median 6 weeks), and the mean time to BOR assessment was 11 weeks (median 7 weeks).