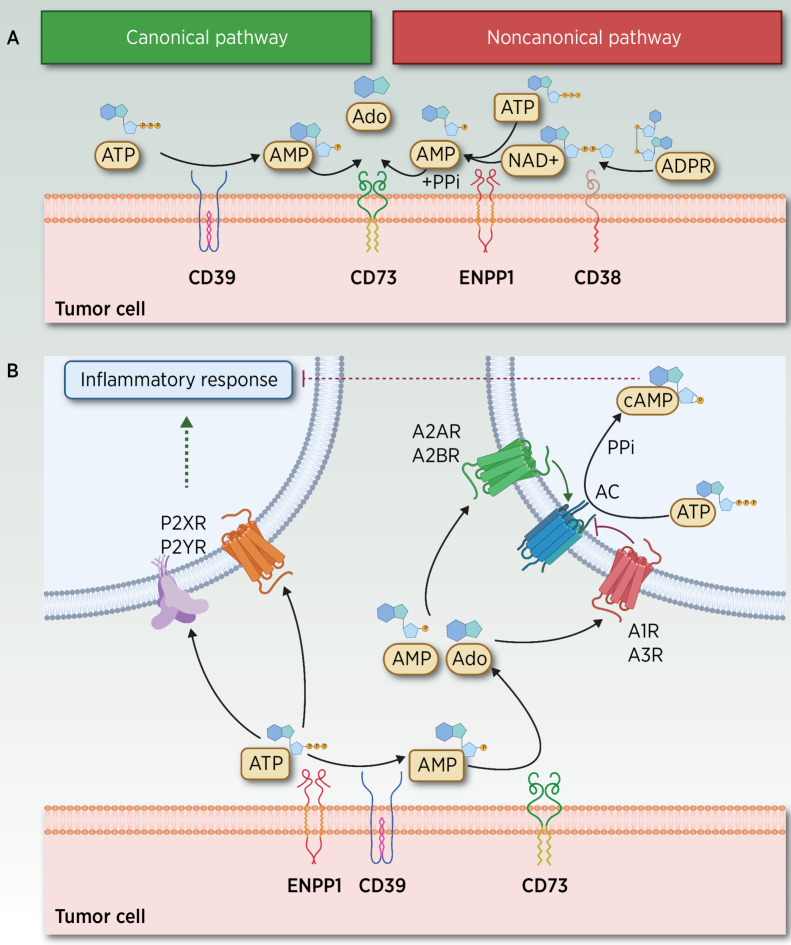
Figure 1.
ATP–Ado extracellular metabolism and elicited signaling through adenosinergic receptors. A, Canonical and noncanonical extracellular receptors contributing to the hydrolysis of ATP and other metabolites. In the TME, two different yet partially overlapping pathways mediate the hydrolysis of ATP to adenosine (Ado). In the noncanonical pathway, Ado can be released by CD38 (72), a cell-surface enzyme that functions as an adhesion molecule and as an ectoenzyme, expressed in T cells, neutrophils, lymphocytes and monocytes, and macrophages under inflammatory conditions. CD38 hydrolyzes nicotinamide-adenine dinucleotide (NAD+) to ADP-ribose (ADPR), which is subsequently metabolized to AMP by ENPP1, whereas AMP is further dephosphorylated by CD73 to Ado and PPi (73, 74). ENPP1 is a Ca2+ and Zn2+–dependent enzyme comprising two identical disulfide bonded subunits (75) involved in the regulation of hormonal (39), neurologic, immunologic, and hematologic functions (4, 76, 77) as well as in atheromatous plaque calcification (78). ENPP1 hydrolyzes its most suitable substrate ATP to AMP, and the catalysis of GTP to GMP while releasing PPi. Other substrates include dinucleotides, mainly cGAMP, diadenosine tetraphosphate, and other poor substrates (UTP and cAMP). The product AMP is subsequently dephosphorylated by CD73 (Ecto-5´-nucleotidase or NT5E) to Pi and Ado. In addition to the action of nucleotidases, different membrane transporters, which belong to SCL28 and SCL29 families, also regulate the extracellular bioavailability of Ado (6). In the canonical pathway, transmembrane or soluble ectoenzyme CD39, which converts nicotinamide-adenine dinucleotide phosphate (NADP) into NAD+, also hydrolyzes extracellular ATP to AMP (79), a catalytic activity similar to that exerted by ENPP1. CD39 is expressed in the vasculature, in malignant cells, and in several immune subpopulations, including M2-like tumor-associated macrophages (TAM), T regulatory cells (Tregs), dendritic cells (DC), natural killers (NK), monocytes, and B cells. CD39 is expressed in MDSC and Tregs, and it is probably high on tumor-specific clonally expanded Tregs (80, 81) events associated with their immunosuppressive role in T-cell function (82). Ado results from the subsequent CD73-mediated degradation of AMP. Thus, CD73 bridges canonical and noncanonical pathways, a role currently exploited by a targeted therapy (83). B, ATP, AMP, and Ado signaling through adenosinergic receptors. ATP and Ado, together with other related metabolites, signal through purinergic receptors divided into two major families: Ado P1 receptors (A1R/ADORA1, A2AR/ADORA2A, A2BR/ADORA2B, and A3R/ADORA3), whose agonists include AMP and Ado, and P2 receptors, which comprise a family of P2X ionotropic receptors (P2X1–7) stimulated by ATP, and P2Y, which are G protein–coupled metabotropic receptors (P2Y 1, 2, 4, 6, 11–14) activated by nucleotides such as ATP, ADP, UTP, and UDP (84). Under hypoxic conditions, extracellular released Ado levels are further amplified by stimulated CD39–CD73, and by decreased Ado kinase activity, leading to diminished Ado degradation (73). This accumulation of AMP and Ado metabolites stimulates P1 G-protein–coupled A2AR at nanomolar range (also A1R and A3R) and A2BR Ado P1 receptors (at micromolar levels) to elicit intracellular cyclic AMP signaling, resulting in decreased production of proinflammatory cytokines and increased synthesis of anti-inflammatory cytokines (40, 85). AC, adenylyl cyclase; PPi, pyrophosphate. (Adapted from an image created with BioRender.com.)