Abstract
Aims
Remote ischaemic preconditioning (RIPC) is a robust cardioprotective intervention in preclinical studies. To establish a working and efficacious RIPC protocol in our laboratories, we performed randomized, blinded in vivo studies in three study centres in rats, with various RIPC protocols. To verify that our experimental settings are in good alignment with in vivo rat studies showing cardioprotection by limb RIPC, we performed a systematic review and meta-analysis. In addition, we investigated the importance of different study parameters.
Methods and results
Male Wistar rats were subjected to 20–45 min cardiac ischaemia followed by 120 min reperfusion with or without preceding RIPC by 3 or 4 × 5−5 min occlusion/reperfusion of one or two femoral vessels by clamping, tourniquet, or pressure cuff. RIPC did not reduce infarct size (IS), microvascular obstruction, or arrhythmias at any study centres. Systematic review and meta-analysis focusing on in vivo rat models of myocardial ischaemia/reperfusion injury with limb RIPC showed that RIPC reduces IS by 21.28% on average. In addition, the systematic review showed methodological heterogeneity and insufficient reporting of study parameters in a high proportion of studies.
Conclusion
We report for the first time the lack of cardioprotection by RIPC in rats, assessed in individually randomized, blinded in vivo studies, involving three study centres, using different RIPC protocols. These results are in discrepancy with the meta-analysis of similar in vivo rat studies; however, no specific methodological reason could be identified by the systematic review, probably due to the overall insufficient reporting of several study parameters that did not improve over the past two decades. These results urge for publication of more well-designed and well-reported studies, irrespective of the outcome, which are required for preclinical reproducibility, and the development of clinically translatable cardioprotective interventions.
Keywords: Limb remote ischaemic preconditioning, Myocardial ischaemia/reperfusion injury, Three-centre study, Systematic review, Meta-analysis
Graphical Abstract
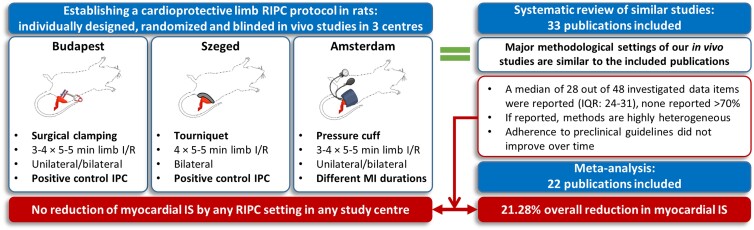
Graphical Abstract.
We tested the cardioprotective efficacy of various RIPC protocols in three study centres in Hungary and the Netherlands. Neither of the applied methodologies resulted in cardioprotection. To verify that our in vivo methodological settings are in accordance with those reported in the literature, a systematic review and meta-analysis was performed. A discrepancy between meta-analysis and our in vivo results was found, despite that major methodological settings were similar to the publications analysed. Systematic review identifies insufficient and highly heterogeneous reporting of the investigated data items that did not improve over time, which could contribute to the discrepancy. RIPC: remote ischaemic preconditioning; I/R: ischaemia/reperfusion; IPC: in situ ischaemic preconditioning; MI: myocardial infarction; IS: infarct size; IQR: inter-quartile range. The figure was created with BioRender.com.
Translational perspective.
Remote ischaemic preconditioning (RIPC) is cardioprotective in preclinical studies; however, its clinical translation has failed, the reasons for which need to be elucidated. Our three-centre rat study shows no cardioprotection by RIPC, which is in discrepancy with our meta-analysis of similar studies. Our systematic review could not identify factors underlying this discrepancy suggesting insufficient reporting and that rat studies on RIPC with the neutral outcome may be missing. We emphasize that the publication of multicentre preclinical studies with rigorous quality control in conducting and reporting, irrespective of their outcome, is essential for preclinical reproducibility, which is necessary for clinical translation.
1. Introduction
Despite the continuous improvement in therapeutic strategies for acute myocardial infarction (AMI), i.e. primary percutaneous coronary intervention and auxiliary pharmacotherapies such as double antiplatelet therapy, the global burden of AMI remains significant.1 Thus, there is still an unmet need for developing novel strategies to reduce myocardial ischaemia/reperfusion (I/R) injury and its long-term consequences to improve the standard of care.2–4
Remote ischaemic conditioning (RIC) is a cardioprotective method that is elicited by short-term, non-lethal cycles of ischaemia and reperfusion to an organ or tissue other than the heart.5–9 RIC can be applied before [remote ischaemic preconditioning (RIPC)], or during myocardial ischaemia [remote ischaemic perconditioning (RIPerC)], or at the beginning of coronary reperfusion (remote ischaemic postconditioning (RIPostC)], showing potential clinical applicability, for example, by cyclic inflation and deflation of a pressure cuff placed on an extremity of the patient.2
There is substantial evidence for the I/R injury-limiting effect of RIC in experimental models, as well as in several, but not all, single-centre clinical studies.10–12 However, RIC techniques were found to be ineffective in robust multicentre trials.13–17 The neutral results seen in these multicentre studies led to the publication of position papers and preclinical guidelines and also the formation of a consortium aiming to increase the translation of cardioprotective therapies from a preclinical perspective.18–22 These studies also provide a basis for critique and a possible explanation of translational problems from the clinical perspective.23,24 Additionally, Rosello and Yellon, as well as the most recent IMPACT COST guideline, described key steps of the optimal translational process, where the very first step is to establish optimal reductionist preclinical models, followed by further experimental investigations to identify novel targets for cardioprotection.25,26
Therefore, to investigate the cardioprotective mechanisms of RIPC, our aim was to establish an efficacious and reliable RIPC protocol in an in vivo rat model of acute myocardial I/R injury in our laboratories. We tested the cardioprotective efficacy of various RIPC protocols by assessing the effect of the number of conditioning cycles, the method of occlusion of femoral vessels, the effector organ mass, and the duration of myocardial ischaemia on the cardioprotective efficacy of RIPC. To reduce systematic or subjective bias in our studies, we performed the in vivo experiments in three study centres in Hungary and the Netherlands, in an individually designed, randomized, and blinded manner.
To verify that our in vivo methodological settings are in accordance with those reported in the literature, and to be able to compare the infarct size (IS)-limiting effect of the current in vivo studies to findings of previous publications, we also performed a systematic review and meta-analysis focusing on studies investigating the cardioprotective efficacy of RIPC in in vivo rat models of acute myocardial I/R injury. In addition, we aimed to investigate the importance of study parameters and methodological settings on outcome and reproducibility.
2. Methods
2.1. Animals and materials
Experiments were performed at three different study centres: Semmelweis University, Budapest (Hungary); University of Szeged (Hungary); and Academic Medical Centre, University of Amsterdam (The Netherlands). At all three study centres, experiments were designed and conducted independently. In all study centres, young (8–10 weeks old) healthy male Wistar rats weighing 250–370 g were kept for 5–21 days of acclimatization in the animal facility of each study centre. In the Budapest and Szeged study centres, animals were obtained from Toxi-Coop Zrt. (Budapest, Hungary). In the Amsterdam study centre, animals were obtained from Charles River, Germany. Rats were housed under controlled temperature (25 ± 2°C) and constant light cycle (12 h light/dark) and allowed free access to a standard rat chow diet and water. Animals were not fasted before surgery.
This investigation complies with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996) and with the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. In the Budapest and Szeged study centres, investigations were compliant with local directives and approved by The Animal Ethics Committees at Semmelweis University, Budapest, and the University of Szeged, Szeged. For the Amsterdam study centre, the study was approved by the Animal Ethics Committee of the Academic Medical Centre, Amsterdam. Unless otherwise noted, all chemicals were purchased from Sigma (St. Louis, MO, USA).
2.2. Study design
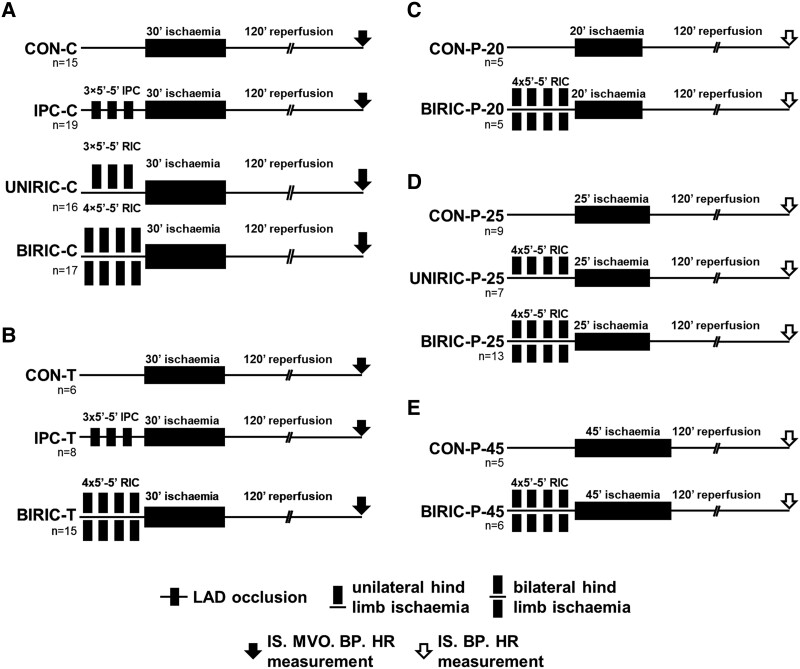
Methodological settings regarding RIPC timing, RIPC occlusion/reperfusion protocol, involved limbs, and techniques of RIPC occlusions, as well as methods of anaesthesia, and length of cardiac index ischaemia were established based on a non-systematic review of the literature performed in April 2018. Animals were randomized sequentially into experimental groups at each individual centre. Coronary ligation and RIPC/sham manoeuvres were performed by independent operators, leading to a blinded application of RIPC, and results were evaluated in a blinded manner at all three study centres. The study design and protocols are illustrated in Figure 1.
Figure 1.
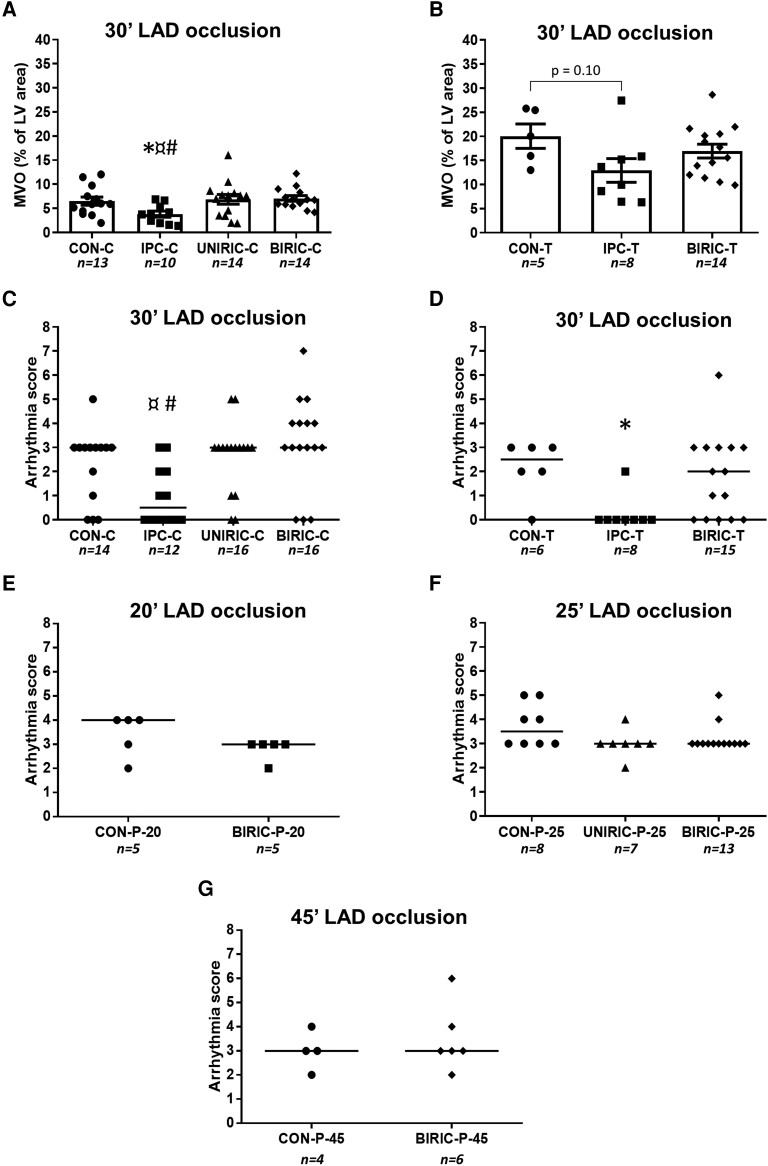
Experimental protocols of myocardial I/R injury and various ischaemic conditioning methods in rats. (A) Budapest study centre—hind-limb ischaemia and reperfusion by clamping femoral artery and vein. (B) Szeged study centre—hind-limb ischaemia and reperfusion by tightening and loosening of a tourniquet. (C–E) Experimental protocol with various durations of myocardial ischaemia in the Amsterdam study centre—hind-limb ischaemia and reperfusion by using pressure cuff. Initial group sizes (n) as the number of animals are shown under the corresponding groups. LAD: left anterior descending coronary artery; RIC: remote ischaemic conditioning; CON: control; IPC: ischaemic preconditioning; UNIRIC: unilateral RIC; BIRIC: bilateral RIC; IS: infarct size; MVO: microvascular obstruction; BP: blood pressure; HR: heart rate.
At the Budapest study centre (Figure 1A), a total of 67 animals were subjected to 30 min index myocardial ischaemia followed by 120 min reperfusion. The control group (CON-C, n = 15) did not receive ischaemic conditioning. The positive control group (IPC-C, n = 19) was subjected to cardiac ischaemic preconditioning (IPC), elicited by 3 cycles of 5 min left anterior descending coronary artery (LAD) occlusion followed by 5 min reperfusion prior to index myocardial ischaemia. The UNIRIC-C group (n = 16) received 3 cycles of unilateral RIC, and the BIRIC-C group (n = 17) received 4 cycles of bilateral RIC by 5 min clamping of femoral artery and vein followed by 5 min hind-limb reperfusion before index myocardial ischaemia (indicated as ‘-C’ in group name).
At the Szeged study centre (Figure 1B), a total of 29 animals were subjected to 30 min myocardial ischaemia followed by 120 min reperfusion. Similar to the study centre of Budapest, the control group (CON-T, n = 6) did not receive ischaemic conditioning, and the positive control group (IPC-T, n = 8) was subjected to IPC according to the same protocol. BIRIC-T group (n = 15) received 4 cycles of bilateral RIC before index myocardial ischaemia by 5 min tightening of a tourniquet on the proximal part of both hind limbs followed by 5 min reperfusion induced by loosening of the tourniquet (indicated as ‘-T’ in group names).
At the Amsterdam study centre (Figure 1C–E), a total of 50 animals were subjected to 20, 25, or 45 min of myocardial index ischaemia (indicated as ‘-20’ or ‘-25’ or ‘-45’ in group names) followed by 120 min reperfusion. Control groups (CON-P-20, n = 5; CON-P-25, n = 9; and CON-P-45, n = 5) did not receive ischaemic conditioning. UNIRIC-P-25 group (n = 7) was subjected to unilateral RIC, whereas BIRIC-P-20 (n = 5), BIRIC-P-25 (n = 13), and BIRIC-P-45 (n = 6) groups were subjected to bilateral RIC by 4 cycles of 5 min inflation of pressure cuffs to 240 mmHg, applied on the proximal part of one or both hind limbs, followed by 5 min reperfusion by deflating pressure cuffs (indicated as ‘–P’ in group names).
At the Budapest and Szeged study centres, stabilization before applying myocardial ischaemia was 40 min, whereas at the Amsterdam study centre, stabilization time was 60 min. The time between the end of the last RIPC or local IPC stimulus and the myocardial index ischaemia was 5 min at all study centres. At all study centres, the presence of hind-limb ischaemia was verified by apparent pallor during ischaemia and pronounced hyperaemia after reperfusion. Following the 120 min myocardial reperfusion, animals were sacrificed humanely under anaesthesia, and hearts were excised for further analysis. The primary endpoint was myocardial infarct size as a percentage of area at risk (IS/AAR) and secondary endpoints were microvascular obstruction (MVO) and I/R-induced arrhythmias.
2.3. Surgical preparation
At the Budapest and Szeged study centres, experimental animals were anesthetized by intraperitoneal (i.p.) injection of pentobarbital sodium (60 mg/kg body weight; Euthasol 20%, Produlab Pharma, Raamsdonksveer, The Netherlands), and anaesthesia was maintained by supplying half dose pentobarbital i.p. as required when the plantar reflex could be elicited through regular paw pinch monitoring. After orotracheal intubation, rats were ventilated with a rodent ventilator (Ugo-Basile, Gemonio, Italy) with room air at a volume of 6.2 mL/kg and frequency of 69 ± 3 breaths/min.
In the Amsterdam study centre, anaesthesia was induced by i.p. injection of pentobarbital sodium (80 mg/kg body weight; Euthasol 20%, Produlab Pharma, Raamsdonksveer, The Netherlands) and maintained by continuous tail vein i.v. infusion at a rate of 30 mg/kg body weight/h. Following intubation, animals were pressure-control ventilated with 35% oxygen in room air at a frequency of 65 breaths/min. The plantar reflex was monitored regularly for depth of anaesthesia.
The following vital parameters were monitored throughout the whole protocol in each study centre: surface electrocardiogram (ECG) was recorded using standard needle limb electrodes (AD Instruments, Bella Vista, Australia); mean arterial blood pressure (MAP) was measured directly by carotid artery cannulation; core body temperature was recorded and maintained by rectal thermometer and heating pad (Harvard Apparatus, Holliston, MA) in Hungary, and by heating pad plus heating lamp in Amsterdam. Body temperature was maintained at physiological temperature (range 37.0–37.5°C). At the Amsterdam study centre, the right jugular vein was cannulated for administration of saline with 20 mM sodium bicarbonate at a rate of 10 mL/kg/h.
Myocardial I/R injury was induced after left minimally invasive thoracotomy. Hearts were exposed and 5–0 Prolene sutures (Ethicon, Johnson & Johnson, Budapest, Hungary) were placed around the proximal part of the LAD, and reversible myocardial ischaemia was induced by tightening a snare around the LAD. At all study centres, the presence of myocardial ischaemia was confirmed by the appearance of ST-segment changes, I/R-induced arrhythmias, and visible pallor of the myocardial regions distal to the occlusion.
After various durations of LAD occlusion, 120 min of reperfusion was induced by relieving the snare. Reperfusion was confirmed by ST-segment normalization, occurrence of early reperfusion arrhythmias, and conspicuous hyperaemia of the reperfused cardiac region. To prevent coagulation, heparin (Budapest study centre: i.p. 100 U/kg; Szeged study centre: i.v. 100 U/kg; Amsterdam study centre: i.v. 25 U/animal) was administered either within 5 min before the beginning of limb ischaemia, at the end of LAD ischaemia, and at the end of reperfusion (centres in Hungary) or at the start of operation only (centre in Amsterdam).
2.4. IS measurement
After 120 min of reperfusion, euthanasia was performed by the excision of the heart under deep anaesthesia. Hearts were immediately perfused retrogradely through the ascending aorta with oxygenated Krebs–Henseleit solution at 37°C on a Langendorff apparatus. After 2 min of equilibration time, the LAD was reoccluded and the area at risk (AAR) was negatively stained by retrogradely perfusing Evans Blue dye through the ascending aorta. Hearts were beating during dye injection. Hearts were then sliced in a standard slice block, giving 2 mm thick contiguous cardiac slices in all study centres, resulting in a total of 6–11 cardiac slices per animal. Viable myocardial tissue was assessed by incubation of cardiac slices in 1% triphenyl tetrazolium chloride (TTC) at 37°C. Hearts were not frozen prior to TTC staining. In the Amsterdam study centre, the basal sides and, in the Budapest and Szeged study centres, both sides of the contiguous cardiac slices were scanned and the area of the necrotic tissue, i.e. the ISs (as proportions of AARs in %) and AARs (as proportions of total left ventricular areas in %), was measured with computer planimetry by independent and blinded investigators using InfarctSize software (version 2.4b, Pharmahungary Group, Szeged, Hungary) in the Budapest and Szeged study centres, or SigmaScan Pro 5 (Systat software, San Jose, CA) in the Amsterdam study centre, as described in previous publications of the workgroups.27–35
2.5. LDH measurement
At the Amsterdam study centre, after 120 min reperfusion, 1 mL blood was obtained from the carotid artery, followed by immediate centrifugation (3 min at 13 400 rpm), and the supernatant plasma was stored at −80°C for further analysis. Lactate dehydrogenase (LDH) activity was determined spectrophotometrically at 25°C, with pyruvate and nicotinamide adenine dinucleotide hydrogen (NADH). The formation of NAD+ from NADH was determined over 3 min to obtain LDH activity.
2.6. MVO measurement
MVO was measured in the Budapest and Szeged study centres. Retrogradely perfused hearts were stained with Thioflavin-S Fluorescent dye immediately prior to the administration of Evans Blue dye. Heart slices were put into a dark chamber and high-resolution photos were taken under ultraviolet light. The size of MVO was estimated by computer planimetry using ImageJ software (version 1.51j8, NIH, USA) and expressed as the proportion of the total left ventricular area.
2.7. Arrhythmia analysis
The severity and duration of I/R-induced arrhythmias were analysed by independent investigators in a blinded fashion. Continuous ECG records of each animal were scored according to the Lambeth conventions and quantified as previously described by Curtis et al.36,37 To increase the time resolution of the occurrence of arrhythmias, each of the ECG records was divided into 5 min intervals and every interval was individually scored according to the most severe arrhythmia type using the above-mentioned scoring system.
2.8. Mean arterial pressure and heart rate measurement
Blood pressures and heart rates (HRs) were averaged in 5 min intervals throughout the whole protocol.
2.9. Mortality analysis
The cause of death was classified as irreversible ventricular fibrillation (VF), pulseless electrical activity, or bradycardia (<150 BPM), accompanied by hypotension (MAP < 15 mmHg). After suspecting life-threatening events during monitoring, attempts were made to resuscitate animals by tapping or flicking the chest, followed by chest compressions at a regular, near-physiological frequency. If the life-threatening event was irreversible within 5 min, the animal was considered to be dead and excluded from further analysis (see Results for exclusion criteria).
2.10. Systematic review
We performed a systematic review aiming to verify that the study parameters of the current in vivo studies are in good alignment with previously published in vivo rat studies of acute myocardial I/R injury showing cardioprotection by limb RIPC. We assessed the reporting frequency of methodological parameters and their values. The systematic review was not registered.
The systematic literature search was performed in accordance with the PRISMA guidelines38 and was conducted on 23 April 2021 by N.V.S., H.T., and V.Z. Two different search terms were used to identify articles of interest in PubMed, details of which are available in the Supplementary material online, in the Search Strategy section. Further studies were identified by consulting with experts in the field.
The Population, Intervention, Comparison, Outcomes and Study (PICOS) approach was used to define study eligibility criteria,38 aiming to find original research articles investigating cardioprotection by limb RIPC compared with control (sham procedure or no treatment) in in vivo rat models of acute myocardial I/R injury, measuring IS/AAR by TTC staining. A detailed breakdown of the PICOS approach can be found in the Supplementary material online, in the Search Strategy section.
Articles were excluded according to the following criteria: in vivo myocardial I/R injury was not performed; RIPC was not performed; RIPC was not elicited by limb I/R; RIPC and myocardial I/R injury were performed in separate animals; no IS/AAR measurement was performed by TTC staining; the article was not available in English; the article was published before 1993, the year of first publication on RIPC. Reviews and editorial letters were also excluded.
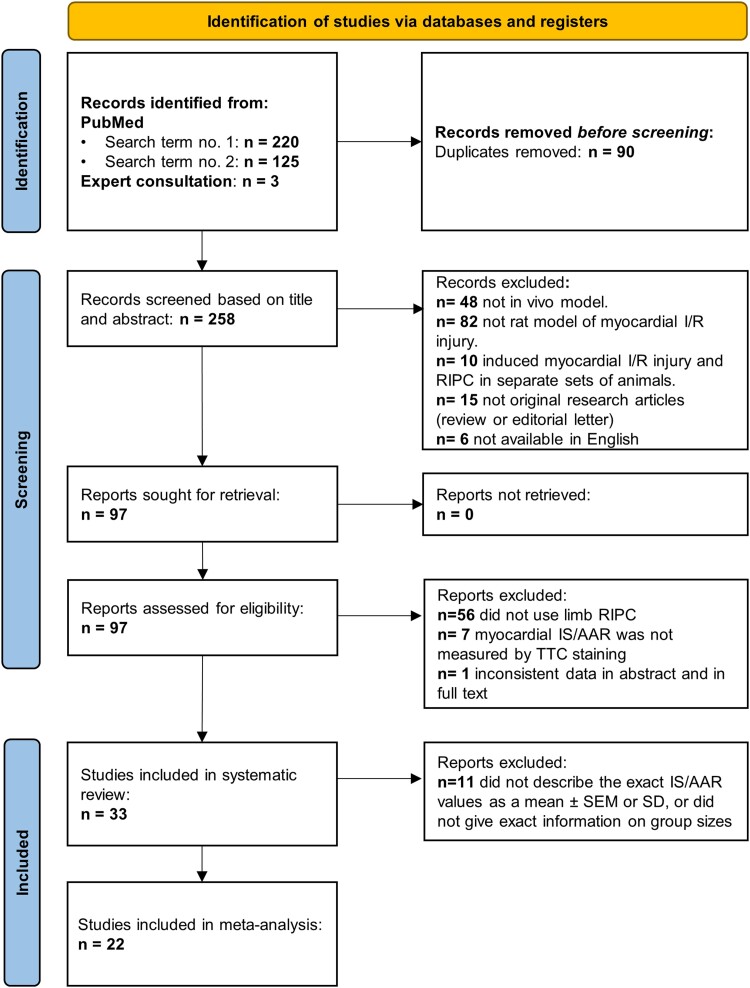
After excluding duplicates, titles and abstracts were screened for eligibility criteria, followed by full-text analysis. The study selection process is summarized in Figure 2. Assessment of eligibility was performed independently in a standardized, unblinded fashion by H.T. and V.Z., and was peer reviewed by N.V.S. Disagreements between reviewers were resolved by consensus or by consulting with senior authors.
Figure 2.
Flow chart of the study selection process. A total of 348 studies were identified by systematic literature search. After excluding 90 duplicates, a total of 225 studies were excluded after title and abstract and full-text screening, resulting in 33 studies included in the systematic review and 22 articles in the meta-analysis.
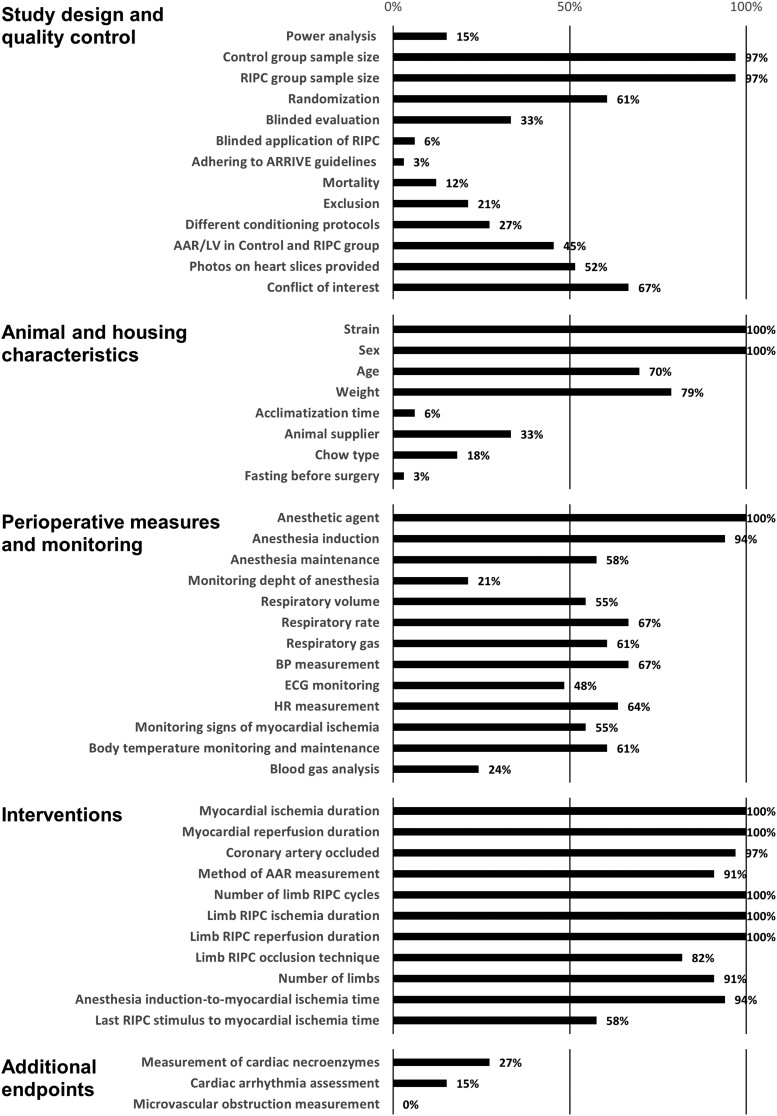
Using a predefined datasheet, data extraction was performed by H.T. and V.Z., and was peer reviewed by N.V.S. Disagreements between reviewers were resolved by consensus or by consulting with senior authors. A total of 56 data items were collated, containing items derived from the ARRIVE guidelines,39 and an extensive list of methodological parameters, i.e. animal and housing characteristics, perioperative measures and monitoring, interventional details regarding RIPC and MI, and endpoints additional to IS/AAR. Forty-eight of these data items were additionally investigated as follows: for every included study, each data item was scored individually in a binary manner by giving either 0 if not reported or 1 if reported. The sum of the individual reported data items per study divided by the total number of reportable data items and the number of studies reporting on each individual data item divided by the total number of studies were calculated. The full list of data items and corresponding data collection principles are available in the Supplementary material online, in the Data items section (see Supplementary material online, Table S1). A full breakdown of the scored and non-scored data items is available in Supplementary material online, Table S2.
2.11. Meta-analysis and risk of bias measurement
The aim of the meta-analysis was to determine the overall cardioprotective efficacy of RIPC and its correlation with the number of reported data items, as well as to assess publication bias. The primary outcome of the current meta-analysis was defined as the unstandardized, weighted mean differences (MDs) between IS/AAR% of the RIPC and control groups. MD was used as all data extracted from the included studies were presented in the same units (IS/AAR%) and measured in a similar manner, i.e. by TTC staining. Articles not describing the exact IS/AAR% as mean ± standard error of the mean (SEM) or mean ± standard deviation (SD), or lacking exact description of group sizes were excluded from the meta-analysis.
For the independent comparisons, effect sizes as MDs, and the 95% confidence intervals (CI) were used. Heterogeneity was assessed by the I2, τ2 statistics and test of heterogeneity. As the included studies were found to be highly heterogeneous, random-effects DerSimonian–Laird model was used for the analysis. To test the robustness of the current meta-analysis, sensitivity was analysed by re-performing the meta-analysis using normalized mean difference (NMD, the MD divided by the mean value in the control group).
To assess whether the number of reported data items influences the effect size, a random-effects meta-regression was performed. Publication bias was assessed by visual interpretation of the funnel plot for asymmetry, the use of Egger’s regression test for assessing small study effects, and non-parametric trim-and-fill analysis.
2.12. Statistical analysis
Continuous data (i.e. IS/AAR, MVO, 5 min arrhythmia scores, mean arterial pressure, and HR values) are shown as mean ± SEM, and differences between ≥3 groups were evaluated using one-way analysis of variance followed by Fisher’s least significant difference (LSD) post hoc test for multiple comparisons, whereas in the case of 2 groups, differences were evaluated using unpaired Student’s t-test. Discrete values of arrhythmia scores are shown as median (25 and 75% inter-quartile range) and differences between ≥3 groups were evaluated using the Kruskal–Wallis test, followed by Dunn’s post hoc test with multiple comparisons, and in the case of 2 groups, differences were evaluated using the Mann–Whitney test. Statistical analysis was performed using GraphPad Prism software (version 6.01, San Diego, USA). For all the statistical tests, the level of significance was set at P < 0.05. For the meta-analysis, Egger’s regression test, and the non-parametric trim-and-fill analysis, STATA 16.1 software was used.
3. Results
3.1. Limb RIPC does not affect myocardial IS in the current in vivo rat studies
We aimed to establish a limb RIPC protocol in an in vivo rat model of myocardial I/R injury with an IS-limiting efficacy similar to that of the literature, as a first step of further studies in our laboratories. Studies were conducted in three study centres and were designed and performed independently in an individually blinded and randomized fashion, with local variations in experimental parameters and techniques in the three study centres consistent with the range of approaches and variations recorded in the published literature.
In the in vivo experiments, animals were excluded from further evaluation either due to death during the experiment, unsuccessful recording of ECG during the whole protocol, lack of ST-segment elevation or depression during myocardial ischaemia, or technical failure at Evans Blue staining (1 animal in the CON-C group; 7 animals in the IPC-C group; 1 animal in the BIRIC-C group; 1 animal from CON-P-25; 1 animal from the CON-P-45 group). Animals were excluded from the IS/AAR measurement, but not from the arrhythmia analysis due to death after randomization (1 animal in the CON-C group; 2 animals in the IPC-C group; 2 animals in the UNIRIC-C group; 2 animals in the BIRIC-C group; 1 animal in the CON-T group; 1 animal in the BIRIC-T group; 1 animal in the BIRIC-P-45 group). Mortality rates (as % of group sizes after exclusion) did not differ significantly between experimental groups or study centres (Table 1).
Table 1.
Mortality rates shown as % of initial group sizes
| Experimental group | Initial group sizes (n) | Mortality (% of initial group sizes) |
|---|---|---|
| CON-C | 15 | 7.14% |
| IPC-C | 19 | 16.67% |
| UNIRIC-C | 16 | 12.5% |
| BIRIC-C | 17 | 12.5% |
| CON-T | 6 | 16.67% |
| IPC-T | 8 | 0% |
| BIRIC-T | 15 | 6.67% |
| CON-P-20 | 5 | 0% |
| BIRIC-P-20 | 5 | 0% |
| CON-P-25 | 9 | 0% |
| UNIRIC-P-25 | 7 | 0% |
| BIRIC-P-25 | 13 | 0% |
| CON-P-45 | 5 | 0% |
| BIRIC-P-45 | 6 | 16.67% |
Neither IPC nor RIPC affected mortality rates in any setting. χ2 test was applied for each experimental group.
CON: control; IPC: ischaemic preconditioning; UNIRIC: unilateral RIC; BIRIC: bilateral RIC; RIC: remote ischaemic conditioning. Group sizes (n) as the number of animals are shown under the corresponding column.
IS/AAR was measured to explore the cardioprotective effects of different ischaemic conditioning protocols. Sizes of the area distal to LAD occlusion (i.e. AAR) did not differ significantly between corresponding groups, except for the IPC-C group, where AAR showed a significant decrease compared with the CON-C group (Table 2).
Table 2.
AAR as % of LV areas, and ISs as % of AARs and ISs as % of LV areas
| Experimental group | Group sizes (n) | AAR (% of LV area) | IS/AAR (%) | IS/LV (%) |
|---|---|---|---|---|
| CON-C | 13 | 42.99 ± 2.0 | 58.15 ± 2.14 | 25.03 ± 1.56 |
| IPC-C | 10 | 35.68 ± 2.8*# | 23.45 ± 1.48*¤# | 8.24 ± 0.55*¤# |
| UNIRIC-C | 14 | 40.07 ± 1.9 | 53.12 ± 4.11 | 21.78 ± 2.63 |
| BIRIC-C | 14 | 43.80 ± 1.9 | 55.41 ± 3.60 | 24.31 ± 1.79 |
| CON-T | 5 | 37.43 ± 3.8 | 43.43 ± 5.08 | 17.60 ± 2.16 |
| IPC-T | 8 | 39.17 ± 8.1 | 20.56 ± 3.91*# | 8.25 ± 2.05*# |
| BIRIC-T | 14 | 42.37 ± 4.1 | 44.87 ± 5.84 | 23.17 ± 2.61 |
| CON-P-20 | 5 | 31.59 ± 6.1 | 48.31 ± 10.46 | 16.40 ± 4.93 |
| BIRIC-P-20 | 5 | 32.09 ± 4.5 | 56.27 ± 7.07 | 17.60 ± 3.23 |
| CON-P-25 | 8 | 30.48 ± 3.7 | 59.60 ± 7.25 | 17.13 ± 3.28 |
| UNIRIC-P-25 | 7 | 34.97 ± 4.3 | 55.10 ± 6.60 | 17.96 ± 1.82 |
| BIRIC-P-25 | 13 | 31.00 ± 2.2 | 61.84 ± 3.71 | 19.83 ± 1.58 |
| CON-P-45 | 4 | 34.93 ± 3.4 | 80.34 ± 5.33 | 26.72 ± 3.13 |
| BIRIC-P-45 | 5 | 36.90 ± 3.8 | 72.29 ± 2.70 | 27.03 ± 2.89 |
Results are presented as mean ± SEM. In cases of ≥3 groups, one-way ANOVA and uncorrected Fisher’s LSD post hoc tests were used. *P < 0.05 vs. CON, ¤P < 0.05 vs. UNIRIC, #P < 0.05 vs. BIRIC. In cases of 2 groups, unpaired t-tests were used.
AAR: area at risk; LV: left ventricle; CON: control; IPC: ischaemic preconditioning; UNIRIC: unilateral RIC; BIRIC: bilateral RIC; RIC: remote ischaemic conditioning. Group sizes (n) as the number of animals are shown under the corresponding column.
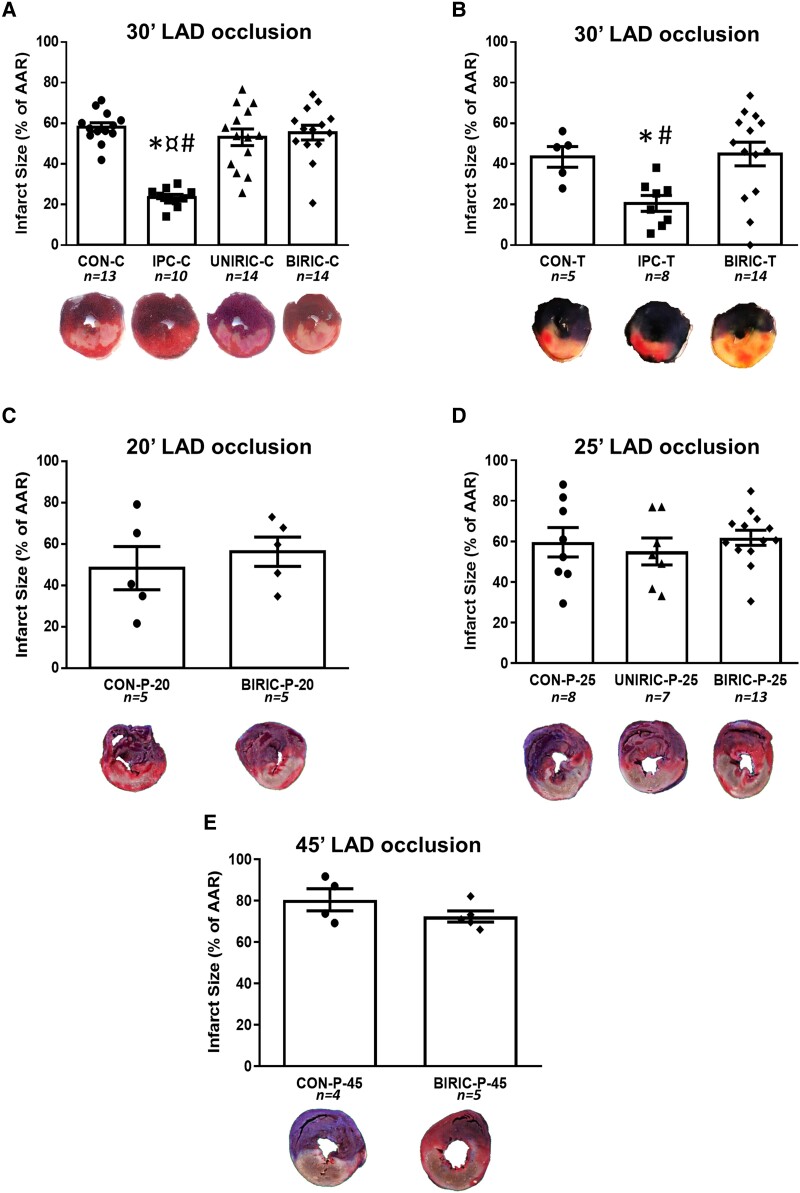
At the Budapest study centre, RIPC performed by cyclic clamping and releasing of femoral vessels either uni- or bilaterally did not reduce IS/AAR (53.12 ± 4.11% and 55.41 ± 3.60% in UNIRIC-C and BIRIC-C groups, respectively), whereas, in the positive control IPC-C group, IS/AAR showed a significant reduction compared with the CON-C group (23.45 ± 1.48% and 58.15 ± 2.14%, respectively) (Figure 3A, Table 2).
Figure 3.
Myocardial ISs as % of the area at risk (IS/AAR). Results are presented as mean ± SEM. (A) Budapest study centre—one-way analysis of variance (ANOVA) and uncorrected Fisher’s LSD post hoc test. (B) Szeged study centre—one-way ANOVA and uncorrected Fisher’s LSD post hoc test. (C–E) Amsterdam study centre—experimental protocol with various durations of myocardial ischaemia, and in cases of C and E, unpaired t-test. In the case of D, one-way ANOVA, uncorrected Fisher’s LSD post hoc test, *P < 0.05 vs. CON, ¤P < 0.05 vs. UNIRIC, #P < 0.05 vs. BIRIC. LAD: left anterior descending coronary artery; CON: control; IPC: ischaemic preconditioning; UNIRIC: unilateral RIC; BIRIC: bilateral RIC; RIC: remote ischaemic conditioning. Group sizes (n) as the number of animals are shown under the corresponding groups.
At the Szeged study centre, RIPC affected by cyclic tightening and loosening of bilateral tourniquets (in the BIRIC-T group) did not decrease IS/AAR, whereas, in the positive control IPC-T group, ∼53% relative decrease in IS/AAR was shown when compared with CON-T (44.87 ± 5.84%, 20.56 ± 3.91%, and 43.43 ± 5.08% in the BIRIC-T, IPC-T, and CON-T groups, respectively) (Figure 3B, Table 2).
At the Amsterdam study centre, RIC was elicited by cyclic inflation and deflation of unilateral or bilateral pressure cuffs applied on hind limbs. Neither unilateral RIC (in UNIRIC-P-20, UNIRIC-P-25, and UNIRIC-P-45 groups) nor bilateral RIC (in the BIRIC-P-25 group) influenced IS/AAR when performed before 20, 25, or 45 min of myocardial ischaemia compared with corresponding controls (CON-P-20, CON-P-25, and CON-P-45, respectively) (Figure 3C–E, Table 2). Further, RIPC did not decrease cardiac necroenzyme levels compared with CON at the Amsterdam study centre (see Supplementary material online, Figure S1). IS/LV data of all studies show similar results to that of IS/AAR data (Table 2).
3.2. Limb RIPC does not affect MVO and arrhythmia scores in the current in vivo rat studies
To further examine the severity of myocardial I/R injury, the extent of MVO was measured at the Budapest and Szeged study centres. While the positive control IPC-C significantly decreased the extent of MVO and in IPC-T MVO tended to be lower than in control groups, none of the different RIC protocols used in any of the study centres showed a reduction in MVO when compared with corresponding control groups (Figure 4A and B).
Figure 4.
MVO and arrhythmia scores. In the case of MVO, results are presented as mean ± SEM; in the case of arrhythmia scores, results are presented as median. (A) MVO at the study centre of Budapest—one-way ANOVA and uncorrected Fisher’s LSD post hoc test. (B) MVO at the study centre of Szeged—one-way ANOVA, uncorrected Fisher’s LSD post hoc test, *P < 0.05 vs. CON, ¤P < 0.05 vs. UNIRIC, #P < 0.05 vs. BIRIC. (C) Arrhythmia scores at the study centre of Budapest—the Kruskal–Wallis test, multiple comparisons, and Dunn’s post hoc test. (D) Arrhythmia scores at the study centre of Szeged—the Kruskal–Wallis test, multiple comparisons, and Dunn’s post hoc test. (E–G) Arrhythmia scores at the study centre of Amsterdam with various durations of myocardial ischaemia. In cases of E and G, the Mann–Whitney test; in the case of F, the Kruskal–Wallis test, multiple comparisons, and Dunn’s post hoc test. MVO: microvascular obstruction; LV: left ventricle; LAD: left anterior descending coronary artery; CON: control; IPC: ischaemic preconditioning; UNIRIC: unilateral RIC; BIRIC: bilateral RIC; RIC: remote ischaemic conditioning. Group sizes (n) as the number of animals are shown under the corresponding groups.
To measure the effect of different ischaemic conditioning protocols on cardiac I/R-induced arrhythmias during myocardial ischaemia and early reperfusion, arrhythmia analysis was performed according to the Lambeth conventions. At the Budapest study centre, cardiac arrhythmias were not significantly reduced in UNIRIC-C and BIRIC-C groups compared with the CON-C group. In the positive control IPC-C group, arrhythmia scores were significantly lower when compared with UNIRIC-C and BIRIC-C groups and tended to be lower when compared with CON-C (Figure 4C). At the Szeged study centre, the occurrence, severity, and duration of cardiac arrhythmias of the BIRIC-T group did not differ significantly from that of the CON-T group, whereas in the positive control IPC-T group, arrhythmia scores showed a significant reduction when compared with CON-T (Figure 4D). In Amsterdam, arrhythmia scores of UNIRIC-P-20, UNIRIC-P-25, BIRIC-P-25, and UNIRIC-P-45 groups showed no significant difference in comparison with corresponding control groups (CON-P-20, CON-P-25, and CON-P-45, respectively) (Figure 4E–G).
To increase the time resolution of arrhythmias, arrhythmia scores were calculated in each 5 min interval of the entire ischaemic period and the first 15 min of reperfusion. None of the RIC protocols at any study centre showed a significant difference in arrhythmia scores in any 5-min interval when either median or mean values were compared with corresponding control groups. However, cardiac arrhythmias in the IPC-C group were significantly lower in several intervals when compared with CON-C, UNIRIC-C, or BIRIC-C (see Supplementary material online, Figure S2 and Tables S3–S7).
MAP and HR data throughout the whole protocol can be found in Supplementary material online, Figures S3 and S4, and Tables S8–S12.
3.3. Systematic review evidences no difference between the most often reported methodological settings in the literature and the methods used in our in vivo study
In order to identify methodological differences and possible methodological confounding factors underlying the neutral cardioprotective results of limb RIPC seen in the current in vivo experiments, we performed a systematic review of the literature and evaluated the reporting frequencies of key methodological settings. Accordingly, a total of 348 articles were identified by the two search algorithms on PubMed and by consulting with experts, followed by the removal of 90 duplicates. A sum of 258 articles was investigated for eligibility criteria; 161 articles were excluded by title and abstract screening, and an additional 64 articles were excluded by full-text screening, resulting in a total of 33 articles included in the systematic review. The causes of exclusion at each level of eligibility investigation are summarized in Figure 2. Included studies are referenced in the Supplementary material online.
Out of 33 studies investigating the cardioprotective effect of limb RIPC in in vivo rat models of myocardial I/R injury, all studies used male animals, as in our experiments. Fifteen studies used Wistar, 17 studies used Sprague-Dawley, and 1 study used Zucker strain. Since no clear preference for animal strain was seen in the reviewed studies, the use of Wistar rats in our experiments may not be considered as a significant methodological variation.
Rat models of comorbidity were used in 12% of studies: acute or chronic hyperglycaemia, hypercholesterolaemia, or uraemia was modelled in 6%, 3%, and 3% of publications, respectively; 64% of studies investigated the effects of different drugs on the cardioprotective effect of RIPC, either given to interrogate signal transduction or to investigate cardioprotective effects additive to RIPC. Cardioprotective effects of different ischaemic conditioning protocols were compared in 27% of studies. In our in vivo model, we used healthy and young rats without any comorbidities or comedications to avoid their known confounding effect, and at the Budapest and Szeged study centres, IPC was used as a positive control.
In total, 67% of studies used pentobarbital as anaesthetic, 9% used chloral hydrate, 9% used volatile agents (isoflurane or sevoflurane), and 18% used other types of anaesthetic; 6% of studies used either mixed anaesthesia or compared the effect of different anaesthetics (for details, see Supplementary material online, Table S2). In our in vivo experiments, we used pentobarbital anaesthesia in all three centres, as reported by the majority of the reviewed studies. However, high heterogeneity of the induction or maintenance doses and administration sites was found (for details, see Supplementary material online, Table S2).
Out of 33 studies, 15 publications reported on the use of room air ventilation, and 5 described the use of supplementary oxygen; however, the remaining 13 studies did not report on the type of respiratory gas. At the Budapest and Szeged study centres, room air ventilation was used, and in the Amsterdam study centre, oxygen supplementation was applied, with a respiratory rate and volume similar to published studies.
All studies induced myocardial I/R injury by occluding and releasing the LAD (sometimes named as left coronary artery in the rat). In 45.5% of studies, durations of myocardial index ischaemia and reperfusion were 30 and 120 min, respectively, and 6% of studies used recovery models of myocardial I/R injury using a 24 h reperfusion model. Details on durations of corresponding ischaemia and reperfusion are available in Supplementary material online, Table S13. As at the Amsterdam study centre, the experiments were designed to assess the effect of myocardial ischaemia duration on RIPC efficacy, short (20 min), commonly reported (25 min), and long (45 min) durations were used, whereas at the Budapest and Szeged study centres, the most commonly applied, 30 min myocardial ischaemia was performed. All study groups were subjected to 120 min reperfusion in our experiments, as reported in 82% of the reviewed studies.
The following methodological characteristics of limb RIPC were investigated by our systematic review: number of RIPC cycles, number of limbs involved, limb ischaemia duration, limb reperfusion duration, and the technique of establishing limb ischaemia. Twenty-one per cent of studies used 1 cycle, 42% used 3 cycles, 33% used 4 cycles, and 6% used 3 times daily 3 cycles of limb RIPC; 45.5% of studies used unilateral limb ischaemia, 45.5% used bilateral limb ischaemia, but 9% did not report on the number of limbs involved in RIPC. Eighty-two per cent of studies used 5 min limb ischaemia followed by 5 min limb reperfusion, 3% used 10 min limb ischaemia followed by 10 min limb reperfusion, and 15% used 15 min limb ischaemia followed by 10 min limb reperfusion; 52% of studies established limb ischaemia by invasive surgical methods; 30% used non-invasive methods of which 12% were conducted by uncontrolled tightening of the limb using tourniquet, and the remaining 18% used external pressure cuffs. However, 18% of studies did not give precise information on the technique of RIPC. A summary of the corresponding number of ischaemic hind limbs and the number of RIPC cycles is available in Supplementary material online, Table S14.
For the assessment of IS/AAR%, 9 of the 33 studies reported on ex vivo retrograde perfusion of the hearts with the AAR-staining dye, 19 studies stained the hearts in vivo, and 5 studies did not exactly describe the staining method of AAR. As the Langendorff method is frequently used for staining the AAR, here we also used the ex vivo retrograde perfusion method.
During the data collection process, other study parameters were found to be reported with a lower frequency, but if reported, a remarkable heterogeneity between the studies was identified (see Supplementary material online, Table S2). Therefore, we decided to assess the reporting frequencies of study parameters, based on whether a given data item was reported or not.
3.4. Systematic review identifies insufficient reporting in a high proportion of in vivo rat studies on cardioprotective effects of limb RIPC
To enable measurement of the overall reporting of the reviewed studies, the number of reported items of each study was assessed as described in Section 2.10.
All study characteristics collected according to the data items, as well as the number of reported data items in each included study, are available in Supplementary material online, Table S2. The median of the number of reported items was 28 out of 48 (inter-quartile range: 24–31), and the number of reported data items did not increase in correlation with the publication date (see Supplementary material online, Figure S5A).
Reporting frequencies on each parameter are shown in Figure 5, resulting in a notable lack of reporting on animal housing; use of quality control measures, e.g. anaesthetic reflex surveillance and AAR/LV data; and measuring other consequences of myocardial I/R injury such as arrhythmias or MVO. We also measured the distribution of studies with different levels of reported data items (shown in Supplementary material online, Figure S5B), demonstrating that only 30% of the included studies reported 60–70%, but none of them reported more than 70% of the investigated study parameters. These data suggest that the number of reported items in the majority of the reviewed studies is insufficient or inadequate for full evaluation and reproduction.
Figure 5.
Frequencies of reporting a parameter by category. A number of studies reporting on certain parameters are expressed as a percentage of all included studies.
The number of reported data items of 44 was achieved in the Budapest, Szeged, and Amsterdam study centres, resulting in 92% of the scored data items. As no prospective sample size calculation was done in the three study centres, no clear adherence to the ARRIVE guidelines could be stated,39 resulting in the loss of 2 points out of 48. In the Budapest and Szeged study centres, no blood gas analysis and no cardiac necroenzyme measurement were performed, whereas in the Amsterdam study centre, no IPC positive control group was used, and no MVO measurement was conducted.
3.5. Meta-analysis shows an overall IS-limiting effect independently from the number of reported items and showed no significant publication bias in in vivo rat studies on cardioprotective effects of limb RIPC
To be able to compare the IS-limiting effect of RIPC in the current in vivo studies to the findings of previous publications, we conducted a meta-analysis of the reviewed studies. In addition, we assessed the relation between the number of reported data items and effect size using meta-regression. Furthermore, since we could not identify differences between the methodological parameters of our neutral in vivo studies and those of the studies in the literature, the question was raised whether there may be studies with smaller IS-limiting or neutral outcomes regarding limb RIPC withheld from publication. To assess the possibility of this phenomenon, we conducted a publication bias assessment.
From the 33 studies included in the systematic review, only 22 were included in the meta-analysis, as the remaining 11 articles did not describe exact IS/AAR values as a mean ± SEM or SD, or did not give exact information on group sizes (Figure 2), necessary for meta-analysis. Of these 22 articles, data on 23 controlled comparisons of RIPC in rat models of acute myocardial I/R injury without any comorbidity or comedication were extracted, including a total of 189 animals in the control groups and 188 animals in the RIPC groups. In the case of studies where the effect of different anaesthetics on RIPC efficacy was investigated, only the groups with the reference anaesthetic were included.
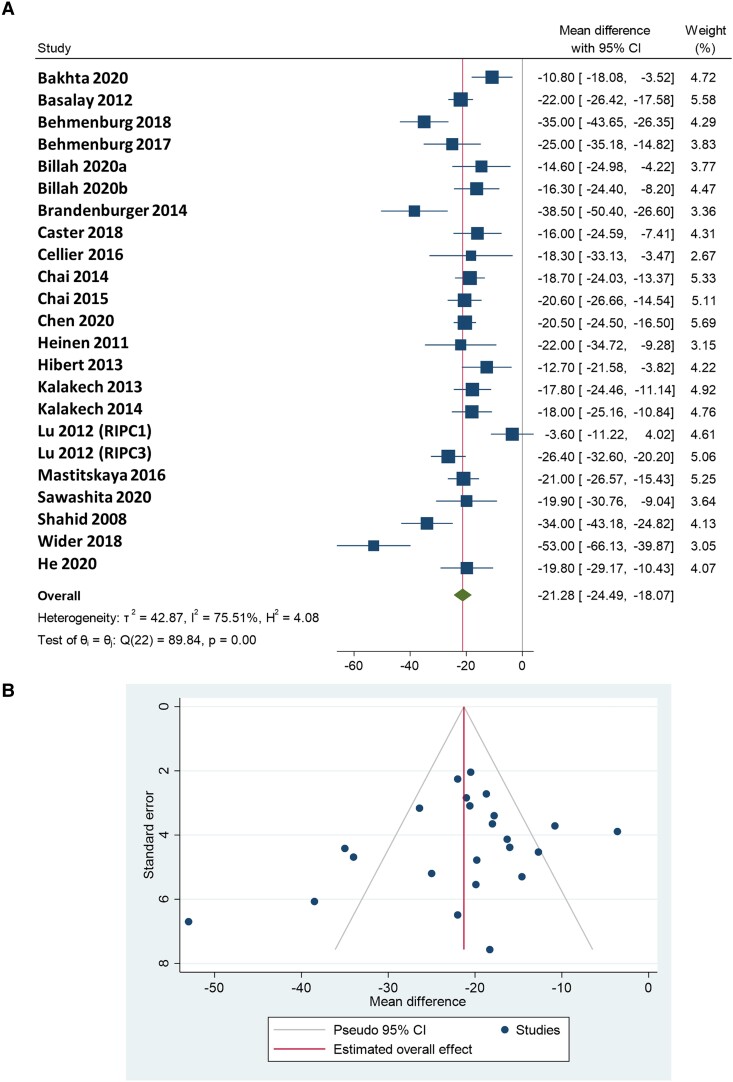
Heterogeneity of the studies was found to be significant (I2 = 75.51% and τ2 = 42.87; P < 0.001). RIPC reduced IS/AAR by 21.28% (95% CI 18.07–24.49) compared with the control group (df = 22; P < 0.00001), as summarized in Figure 6A. By re-performing the analysis using NMD, similar results were obtained, as heterogeneity was observed to be significant (I2 = 49.21% and τ2 = 74.15; P < 0.001), and the overall effect was 34.68 favouring RIPC towards control (95% CI 29.37–39.99). The forest plot using NMD is shown in Supplementary material online, Figure S6.
Figure 6.
(A) Forest plot of the meta-analysis on cardioprotective efficacy (defined as a reduction in IS/AAR%) of RIPC in in vivo rat models of acute myocardial I/R injury, using random-effects DerSimonian–Laird method. A total of 22 controlled comparisons were made, with a total of 194 and 195 animals included in the control and RIPC groups, respectively. (B) Funnel plot for the assessment of publication bias. The vertical line represents the estimated overall mean effect size, and the diagonal lines represent the pseudo-95% CI accordingly. Publication bias was assessed visually, followed by Egger’s regression test and non-parametric trim-and-fill analysis.
We investigated the impact of the number of reported data items on the outcomes by performing meta-regression using the number of reported items as the independent variable and MD as the dependent variable, and found no significant relationship between them [estimated meta-regression coefficient: −0.666 (95% CI: −1.395–0.063); P = 0.07]. The forest plot of the studies ordered by the number of reported items is shown in Supplementary material online, Figure S7.
Publication bias was assessed by visual interpretation of the funnel plot (Figure 6B), suggesting that small studies with small or no cardioprotective efficacy of RIPC may be underrepresented among published results; however, Egger’s regression test showed no significant publication bias (P = 0.07), and the non-parametric trim-and-fill method did not indicate missing studies which would compensate the asymmetry of the funnel plot. The funnel plot using NMD and the result of Egger’s test (P = 0.07) were similar to the analysis using MD. In this case, the trim-and-fill analysis indicated three missing studies to compensate for the asymmetry of the funnel plot (see Supplementary material online, Figure S8).
4. Discussion
Here we performed in vivo rat experiments in an individually designed, blinded, and randomized fashion in three study centres in Hungary and the Netherlands, with methodological settings corresponding to the most commonly reported features of published studies. We demonstrate for the first time no cardioprotective effect of limb RIPC in in vivo rat models of acute myocardial I/R injury, which is in discrepancy with the meta-analysis of similar in vivo rat studies showing robust cardioprotection by RIPC. This discrepancy may be due to the insufficiently reported methodological details and design parameters in the majority of studies, and the high heterogeneity in a number of experimental settings, as identified by the current systematic review. In addition, publications reporting on conditions when RIPC did not work (other than the well-known confounders of comorbidities and comedications) or on methodological details that are crucial for RIPC to be cardioprotective are lacking. Together, these factors hinder reproducibility, which is necessary for successful translation.
To enable the investigation of cardioprotection by RIPC in accordance with Step 1 of the latest IMPACT COST guidelines,26 we aimed to establish a robust and efficacious RIPC protocol in our laboratories with a reductionist study design, as a basis for further investigations in more complex models. The current experiments were conducted independently in three study centres in an individually and independently designed, blinded, and randomized fashion under rigorous quality control, with methodological settings within the boundaries of the published literature. We tested the cardioprotective efficacy of various RIPC protocols by (i) assessing the effect of the number of conditioning cycles, i.e. 3 or 4; (ii) the method of occlusion of femoral vessels, i.e. either surgical clamping of the femoral vessels, or applying uncontrolled pressure on the limbs by tightening a tourniquet around them, or applying controlled pressure on the limbs by using pressure cuffs; (iii) the effector organ mass, i.e. involving 1 or 2 hind limbs; and (iv) the myocardial ischaemia duration, ranging between 20 and 45 min. Surprisingly, our current experiments with RIPC resulted in a neutral outcome, as no reduction in IS, MVO, or I/R-induced arrhythmias could be achieved at any study centre by any RIPC protocol, although the positive control IPC was significantly cardioprotective. It should be mentioned here that in a previous study in the Budapest study centre, cardioprotection by RIPerC had previously been achieved,28 and also that studies with a neutral outcome on RIPerC either combined with RIPostC or alone,40 or in ex vivo RIPC models have already been published;41 however, our present studies are the first to report a neutral effect of limb RIPC on IS in rats in vivo. As our experimental results are in contrast to similar studies, we hypothesized that this discrepancy may be attributed to differences in methodological settings between our present experiments and the previously published ones. To investigate the possible underlying methodological reasons for the observed neutral outcome, as a next step, we reviewed the literature systematically and collated methodological settings in a detailed fashion.
Systematic reviews are of great importance to highlight factors that are likely to influence the outcomes of cardioprotective therapies. The first comprehensive systematic review and meta-analysis was published in 2017 by Bromage et al., who analysed preclinical experiments investigating remote ischaemic pre-, per-, and postconditioning, conducted in both small and large animal models, providing a general overview of the quality of preclinical studies.42 In our systematic review, we focused on in vivo rat studies of RIPC and incorporated an additional 13 studies published after 2017, and significantly extended the range of methodological and design parameters. Here we analysed parameters regarding animal husbandry characteristics, quality control measures and study design, perioperative measures and monitoring, and intraoperative procedures, all of which are pertinent to experimental reproducibility that potentially could influence study outcomes in RIPC experiments. We found that (i) our methodological settings were in line with the reviewed studies, (ii) the majority of the reviewed studies reported 50–60% of the data items, (iii) even when reported, there is significant methodological heterogeneity in the majority of in vivo rat studies, and (iv) the number of reported items did not increase with time despite the preclinical guidelines and recommendations published regularly. Based on these findings, we could not identify any methodological or design factor that potentially explains our neutral experimental results.
Our finding that methodological descriptions lack necessary details, and the lack of standardized parameters in the published papers, may suggest that the reproduction of experiments is compromised.
The omission of the precise and detailed description of methodological settings and results does not reflect the low quality of a study per se, and does not influence the effect size, as shown by our meta-regression. However, the omission of such detail forces laboratories to independently optimize protocols for cardioprotective interventions. This procedure is often challenging, since only final protocols, resulting in positive outcomes, are published, whereas the preliminary studies on the optimization of protocols are not, apart from dose-ranging studies, in which the number of RIPC cycles and the number of involved limbs are investigated. It may also contribute to high methodological heterogeneity and potentially hinder reproducibility of preclinical RIPC studies. As the current systematic review contains major methodological and design parameters of all the in vivo rat experiments assessing cardioprotection by RIPC published so far, our systematic review may be useful for planning preclinical studies in the future, e.g. in terms of identifying commonly used practices, identifying experimental setups that have not been investigated so far, highlighting key criteria to be reported when publishing, as well as sample size calculation.
Our current meta-analysis showed a robust and unanimous IS-limiting effect of RIPC in in vivo rat studies of myocardial I/R injury, and it provided no evidence on risk for publication bias. The discrepancy between the current neutral in vivo results and the overall positive result of the meta-analysis may be due to (i) overall insufficient reporting, especially on methodological details that are crucial for RIPC to be cardioprotective, (ii) high heterogeneity among reported methods, and (iii) the overall lack of adherence to preclinical guidelines that did not improve over time. These factors hinder preclinical reproducibility and the identification of causes underlying neutral results that are in discrepancy with the majority of publications. Therefore, although meta-analyses are considered to represent the highest level of evidence, and their results are key drivers of future studies from bench to bedside, results of the current and previous42 preclinical meta-analyses on RIPC should be interpreted with caution, since they are based on preclinical studies with heterogeneous methodological settings, which are often unreported in sufficient detail.
Based on the findings of our systematic review and meta-analysis, and assuming that similarly to us, other laboratories have also faced difficulties while establishing cardioprotective protocols relying on previous reports, we emphasize that experimental interventional studies in the field of cardioprotection should be published irrespective of outcome (positive or neutral), if they fulfil the criteria of scientific coherence, sufficient quality control in experimental design and execution, and transparent reporting of all detail required to support independent replication. These studies serve as the solid basis for future more complex preclinical studies, as well as for clinical translatability.
Position papers and preclinical guidelines20,26,19 in the field of cardioprotection give recommendations on how to improve preclinical studies, with an aim to increase reproducibility and translatability. Here, we provide data on the current state of reporting, showing no improvement over time in the last decades, despite the guidelines and recommendations being published regularly. In addition, we also provide a novel concept that needs to be considered in the future, i.e. reporting conditions when a cardioprotective intervention does not work, or methodological details that are crucial for an intervention to be cardioprotective is also needed for improving reproducibility and translatability. We emphasize the importance of adhering to these recommendations, i.e. the paramount need for a clear and detailed reporting of methodologies and results; otherwise, the establishment and optimization of a cardioprotective intervention in a laboratory remains challenging, and the underlying reasons for neutral results cannot be identified. To underscore that the majority of study parameters need to be reported in detail, we now provide a review of the data items we collated systematically and assess their potential influence on the outcome (see Supplementary material online, Discussion on the data items section).
4.1. Limitations
A limitation of the present in vivo experiments is the lack of a positive control IPC group at the Amsterdam study centre; however, previously published studies indicate that cardioprotection can be achieved in the model systems at this centre as well. Another limitation is the lack of a prospective power analysis; nevertheless, using the estimate of the effect sizes and standard deviations derived from the literature of the current systematic review, the sample size results in n = 4, suggesting that the current in vivo studies may not be underpowered. However, this approach should be interpreted with caution.43,44 In addition, a considerable variability in the IS/AAR data of the control groups can be observed between the three study sites, despite the similar or same duration of myocardial ischaemia and the consistency of AAR/LV. IS/AAR depends on the individual response of the animals to myocardial I/R injury. This variability in IS/AAR may also be explained by the limitations of a multicentre preclinical study, i.e. the different sources of animals, and procedural differences that cannot be specified in a study protocol. Other possible factors, e.g. circadian rhythm,45 as shown in a mice model of myocardial infarction (MI),46 may also contribute to the variability in IS/AAR. The current in vivo studies were not designed to investigate the possible confounding effect of the circadian rhythm. Of note, none of the studies included in the present systematic review has described the exact time of the induction of MI; thus, this could be another important parameter to analyse in the future.
Regarding the systematic review, a criticism could be raised on the chosen data items. As key methodological parameters that influence the outcome of RIPC studies are so far unknown, the current collection of data items may inherently be incomplete and subjective. However, our set of data items contains those of previous publications,39,42 with a significant extension on the methodological parameters, which we strongly believe, increased the possibility that the key methodological parameters with a potential to influence the efficacy of RIPC are among the ones analysed here.
Regarding the meta-analysis, the lack of subgroup analyses based on each key methodological factor could be interpreted as incompleteness or a limitation. However, since our main goal with the current meta-analysis was to compare the current in vivo results with that of the literature in a quantified fashion, subgroup analyses would reach beyond the scope of our study. Moreover, although it is common practice to approach authors when there is a question regarding missing data or protocols, it is not obligatory according to the PRISMA guidelines, and additionally, obtaining information on individual basis by personal correspondence greatly undermines transparency and reproducibility in itself; therefore, we did not follow this practice in this paper. In addition, it has to be emphasized that Egger’s regression test showed no statistically significant publication bias towards positive results (P = 0.07); however, one cannot exclude the possibility of withholding neutral or negative results from publications, as seen in many other fields of medical science.47–50
A discrepancy may be noted between the current study where RIPC was neutral and our previous study where RIPerC was shown to be cardioprotective.28 This may be due to the difference in timing of the remote stimuli; however, this statement is speculative, since, to the best of our knowledge, no original research article has investigated the differences between the cardioprotective signalling mechanisms of RIPC and RIPerC yet. Nevertheless, RIPC and RIPostC were proved to show differential cardioprotective pathways,51 also emphasizing the presence of time dependence among different RIC protocols. Of note, our previous RIPerC study reported 30 data items of total 48, where missing data items include power analysis, report on AAR sizes, photos on heart slices, and type of respiratory gas, among others.
As an amendment to the Supplementary material online, Discussion on the data items section, the experience level of the investigator also may influence the outcome of the in vivo cardioprotection studies; however, unfortunately, the definition of ‘experienced investigator’ in terms of cardioprotection has never been defined and studied in any of the papers published so far. Of note, the investigators performing LAD ligation for the current study could be considered to be highly experienced.
4.2. Conclusion
In conclusion, we report here for the first time the lack of cardioprotection by RIPC against IS, MVO, and arrhythmias in rats in vivo applying the most commonly used experimental protocols. We assessed the potential of hind-limb RIPC in three, individually randomized and individually analysed, blinded studies, replicating a range of previously reported RIPC protocols and methodologies, supported by a systematic review and meta-analysis of the published literature. The neutral outcome of our study, despite all key methodological factors were in line with that of the reviewed studies, suggests that the current level of reporting rigour may hinder reproduction and the identification of causes leading to neutral results. Therefore, we emphasize the importance of rigorous control measures, and full reporting in a transparent manner, and the publishing of studies even with small IS-limiting, neutral or negative outcomes, as well as the publishing of optimization processes to reach an efficacious protocol. In addition, we emphasize the need for multicentre preclinical studies on investigating cardioprotective therapies, as suggested by the IMPACT COST guidelines and by the CAESAR consortium. Enacting these aspects of preclinical research will support a more comprehensive preclinical assessment of the cardioprotective efficacy of RIPC (and other potential cardioprotective interventions) by enabling systematic review, meta-analysis, and unequivocal experimental replication. All are essential for successful clinical trial design and translation.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
P.F. and C.J.Z. conceived the study. N.V.S., G.B.B., and Z.G. wrote the manuscript. F.P., R.S., G.F.B., and C.J.Z. provided overall supervision and funding. N.V.S., G.B.B., A.M., B.K., C.K., T.G.G., K.G., T.S., P.B., and A.H. collected and analysed data regarding in vivo study. N.V.S., S.G.A., H.T., V.Z., and Z.V. collected and analysed data regarding systematic review and meta-analysis. All authors contributed to manuscript editing.
Supplementary Material
Contributor Information
Nabil V Sayour, Cardiovascular and Metabolic Research Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Üllői út 26, 1085 Budapest, Hungary.
Gábor B Brenner, Cardiovascular and Metabolic Research Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Üllői út 26, 1085 Budapest, Hungary.
András Makkos, Cardiovascular and Metabolic Research Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Üllői út 26, 1085 Budapest, Hungary; MTA-SE System Pharmacology Research Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Budapest, Hungary.
Bernadett Kiss, Cardiovascular and Metabolic Research Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Üllői út 26, 1085 Budapest, Hungary; MTA-SE System Pharmacology Research Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Budapest, Hungary.
Csenger Kovácsházi, Cardiovascular and Metabolic Research Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Üllői út 26, 1085 Budapest, Hungary.
Tamás G Gergely, Cardiovascular and Metabolic Research Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Üllői út 26, 1085 Budapest, Hungary.
Sverre Groever Aukrust, Cardiovascular and Metabolic Research Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Üllői út 26, 1085 Budapest, Hungary.
Huimin Tian, Cardiovascular and Metabolic Research Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Üllői út 26, 1085 Budapest, Hungary.
Viktória Zenkl, Cardiovascular and Metabolic Research Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Üllői út 26, 1085 Budapest, Hungary.
Kamilla Gömöri, Pharmahungary Group, Szeged, Hungary; Department of Pharmacology and Pharmacotherapy, University of Szeged, Szeged, Hungary.
Tamara Szabados, Pharmahungary Group, Szeged, Hungary; Department of Pharmacology and Pharmacotherapy, University of Szeged, Szeged, Hungary.
Péter Bencsik, Pharmahungary Group, Szeged, Hungary; Department of Pharmacology and Pharmacotherapy, University of Szeged, Szeged, Hungary.
Andre Heinen, Institut für Herz- und Kreislaufphysiologie, Heinrich-Heine-Universität Düsseldorf, 40225 Düsseldorf, Germany.
Rainer Schulz, Institute of Physiology, Justus-Liebig University Giessen, Giessen, Germany.
Gary F Baxter, School of Pharmacy and Pharmaceutical Sciences, Cardiff University, Redwood Building, King Edward VII Avenue, Cardiff CF10 3NB, UK.
Coert J Zuurbier, Laboratory of Experimental Intensive Care and Anesthesiology, Department of Anesthesiology, Amsterdam Cardiovascular Sciences, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Zoltán Vokó, Centre for Health Technology Assessment, Semmelweis University, Budapest, Hungary; Syreon Research Institute, Mexikói út 65/A, 1145 Budapest, Hungary.
Péter Ferdinandy, Cardiovascular and Metabolic Research Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Üllői út 26, 1085 Budapest, Hungary; MTA-SE System Pharmacology Research Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Budapest, Hungary; Pharmahungary Group, Szeged, Hungary.
Zoltán Giricz, Cardiovascular and Metabolic Research Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Üllői út 26, 1085 Budapest, Hungary.
Funding
The Ministry for Innovation and Technology in Hungary provided funding to this study under the Thematic Excellence Programme (2020-4.1.1.-TKP2020), the 2020-1.1.5-GYORSÍTÓSÁV call programme (2020-1.1.5-GYORSÍTÓSÁV-2021-00011), the TKP2021-EGA funding scheme (TKP2021-EGA-23), and the Research Excellence Programme (TKP/ITM/NKFIH). This study was also supported by Project no. RRF-2.3.1-21-2022-00003 "National Heart Laboratory, Hungary" implemented with the support provided by the European Union. This study was further supported by the National Research, Development and Innovation Office of Hungary (NKFIA; NVKP-16-1-2016-0017 National Heart Program). The research was further funded by the National Research, Development and Innovation Office of Hungary (NKFIA; VEKOP-2.3.2-16-2016-00002 and VEKOP-2.3.3-15-2017-00016). N.V.S., K.C. and T.G.G. were supported by the Semmelweis 250+ Excellence PhD Scholarship (EFOP-3.6.3-VEKOP-16-2017-00009) and by the Gedeon Richter Excellence PhD Scholarship. C.K. was supported by the National Talent Program of the Ministry of Human Capacities (NTP-NFTÖ-22-B-0200). G.B.B. was supported by EFOP-3.6.3-VEKOP-16-2017-00009 and Richter Gedeon Nyrt. scholarship. A.M. was supported by the ÚNKP-21-4-I-SE-6 New National Excellence Program of the Ministry of Human Capacities. T.Sz. was supported by the Cooperative Doctoral Programme (KDP-2020) of the Ministry for Innovation and Technology. P.B. was supported by the János Bolyai Research Scholarships of the Hungarian Academy of Sciences, the ÚNKP-22-5-SZTE-542 New National Excellence Program of the Ministry of Human Capacities, and the Hungarian National Scientific Research Fund (OTKA-138223). R.S. was supported by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) [Project number 268555672—SFB 1213, Project B05]. Z.G. was supported by a János Bolyai Research Scholarship of the Hungarian Academy of Sciences by the ÚNKP-18-4 New National Excellence Program of the Ministry of Human Capacities and the Hungarian National Scientific Research Fund (K_21-139105).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda-Orjuela CA, Castillo-Rivas J, Catalá-López F, Choi JY, Christensen H, Cirillo M, Cooper L Jr, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C.. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heusch G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 2020;17:773–789. [DOI] [PubMed] [Google Scholar]
- 3. Kloner RA, Brown DA, Csete M, Dai W, Downey JM, Gottlieb RA, Hale SL, Shi J. New and revisited approaches to preserving the reperfused myocardium. Nat Rev Cardiol 2017;14:679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferdinandy P, Andreadou I, Baxter GF, Bøtker HE, Davidson SM, Dobrev D, Gersh BJ, Heusch G, Lecour S, Ruiz-Meana M, Zuurbier CJ, Hausenloy DJ, Schulz R. Interaction of cardiovascular nonmodifiable risk factors, comorbidities and comedications with ischemia/reperfusion injury and cardioprotection by pharmacological treatments and ischemic conditioning. Pharmacol Rev `2023;75:159–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993;87:893–899. [DOI] [PubMed] [Google Scholar]
- 6. Pickard JMJ, Bøtker HE, Crimi G, Davidson B, Davidson SM, Dutka D, Ferdinandy P, Ganske R, Garcia-Dorado D, Giricz Z, Gourine AV, Heusch G, Kharbanda R, Kleinbongard P, MacAllister R, McIntyre C, Meybohm P, Prunier F, Redington A, Robertson NJ, Suleiman MS, Vanezis A, Walsh S, Yellon DM, Hausenloy DJ. Remote ischemic conditioning: from experimental observation to clinical application: report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res Cardiol 2015;110:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hadebe N, Cour M, Lecour S. The SAFE pathway for cardioprotection: is this a promising target? Basic Res Cardiol 2018;113:9. [DOI] [PubMed] [Google Scholar]
- 8. Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol 2015;65:177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heusch G. 25 years of remote ischemic conditioning: from laboratory curiosity to clinical outcome. Basic Res Cardiol 2018;113:15. [DOI] [PubMed] [Google Scholar]
- 10. Gaspar A, Lourenço AP, Pereira MÁ, Azevedo P, Roncon-Albuquerque R, Marques J, Leite-Moreira AF. Randomized controlled trial of remote ischaemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic Res Cardiol 2018;113:415–417. [DOI] [PubMed] [Google Scholar]
- 11. Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sørensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 2010;375:727–734. [DOI] [PubMed] [Google Scholar]
- 12. Verouhis D, Sörensson P, Gourine A, Henareh L, Persson J, Saleh N, Settergren M, Sundqvist M, Tornvall P, Witt N, Böhm F, Pernow J. Effect of remote ischemic conditioning on infarct size in patients with anterior ST-elevation myocardial infarction. Am Heart J 2016;181:66–73. [DOI] [PubMed] [Google Scholar]
- 13. Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med 2015;373:1408–1417. [DOI] [PubMed] [Google Scholar]
- 14. Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Böning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg-Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer-Treschan T, Kienbaum P, Heringlake M, Schön J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K; RIPHeart Study Collaborators.. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med 2015;373:1397–1407. [DOI] [PubMed] [Google Scholar]
- 15. Hausenloy DJ, Kharbanda RK, Møller UK, Ramlall M, Aarøe J, Butler R, Bulluck H, Clayton T, Dana A, Dodd M, Engstrom T, Evans R, Lassen JF, Christensen EF, Garcia-Ruiz JM, Gorog DA, Hjort J, Houghton RF, Ibanez B, Knight R, Lippert FK, Lønborg JT, Maeng M, Milasinovic D, More R, Nicholas JM, Jensen LO, Perkins A, Radovanovic N, Rakhit RD, Ravkilde J, Ryding AD, Schmidt MR, Riddervold IS, Sørensen HT, Stankovic G, Varma M, Webb I, Terkelsen CJ, Greenwood JP, Yellon DM, Bøtker HE; CONDI-2/ERIC-PPCI Investigators.. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): a single-blind randomised controlled trial. Lancet 2019;394:1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Francis R, Chong J, Ramlall M, Bucciarelli-Ducci C, Clayton T, Dodd M, Engstrøm T, Evans R, Ferreira VM, Fontana M, Greenwood JP, Kharbanda RK, Kim WY, Kotecha T, Lønborg JT, Mathur A, Møller UK, Moon J, Perkins A, Rakhit RD, Yellon DM, Bøtker HE, Bulluck H, Hausenloy DJ. Effect of remote ischaemic conditioning on infarct size and remodelling in ST-segment elevation myocardial infarction patients: the CONDI-2/ERIC-PPCI CMR substudy. Basic Res Cardiol 2021;116:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. García del Blanco B, Otaegui I, Rodríguez-Palomares JF, Bayés-Genis A, Fernández-Nofrerías E, del Olmo VV, Carrillo X, Ibáñez B, Worner F, Casanova J, Pueo E, González-Juanatey JR, López-Pais J, Bardají A, Bonet G, Fuertes M, Rodríguez-Sinovas A, Ruiz-Meana M, Inserte J, Barba I, Gómez-Talavera S, Martí G, Serra B, Bellera N, Ojeda-Ramos M, Cuellar H, Valente F, Carmona MÁ, Miró-Casas E, Marsal JR, Sambola A, Lidón RM, Bañeras J, Elízaga J, Padilla F, Barrabés JA, Hausenloy DJ, Ferreira-González I, García-Dorado D.. Effect of COMBinAtion therapy with remote ischemic conditioning and exenatide on the myocardial infarct size: a two-by-two factorial randomized trial (COMBAT-MI). Basic Res Cardiol 2021;116:4. [DOI] [PubMed] [Google Scholar]
- 18. Hausenloy DJ, Erik Botker H, Condorelli G, Ferdinandy P, Garcia-Dorado D, Heusch G, Lecour S, Van Laake LW, Madonna R, Ruiz-Meana M, Schulz R, Sluijter JPG, Yellon DM, Ovize M. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 2013;98:7–27. [DOI] [PubMed] [Google Scholar]
- 19. Bøtker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM, Deshwal S, Devaux Y, Di LF, Di SM, Efentakis P, Femminò S, García-Dorado D, Giricz Z, Ibanez B, Iliodromitis E, Kaludercic N, Kleinbongard P, Neuhäuser M, Ovize M, Pagliaro P, Rahbek-Schmidt M, Ruiz-Meana M, Schlüter KD, Schulz R, Skyschally A, Wilder C, Yellon DM, Ferdinandy P, Heusch G. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol 2018;113:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lecour S, Bøtker HE, Condorelli G, Davidson SM, Garcia-Dorado D, Engel FB, Ferdinandy P, Heusch G, Madonna R, Ovize M, Ruiz-Meana M, Schulz R, Sluijter JPG, Van Laake LW, Yellon DM, Hausenloy DJ. ESC working group cellular biology of the heart: position paper: improving the preclinical assessment of novel cardioprotective therapies. Cardiovasc Res 2014;104:399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones SP, Tang XL, Guo Y, Steenbergen C, Lefer DJ, Kukreja RC, Kong M, Li Q, Bhushan S, Zhu X, Du J, Nong Y, Stowers HL, Kondo K, Hunt GN, Goodchild TT, Orr A, Chang CC, Ockaili R, Salloum FN, Bolli R. The NHLBI-Sponsored Consortium for preclinicAl assESsment of cARdioprotective Therapies (CAESAR): a new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ Res 2015;116:572–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lindsey ML, Bolli R, Canty JM, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Longacre LS, Ripplinger CM, Van Eyk JE, Heusch G. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Hear Circ Physiol 2018;314:H812–H838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lefer DJ, Marbán E. Is cardioprotection dead? Circulation 2017;136:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heusch G, Gersh BJ. ERICCA and RIPHeart: two nails in the coffin for cardioprotection by remote ischemic conditioning? Probably not! Eur Heart J 2016;37:200–202. [DOI] [PubMed] [Google Scholar]
- 25. Rossello X, Yellon DM. Cardioprotection: the disconnect between bench and bedside. Circulation 2016;134:574–575. [DOI] [PubMed] [Google Scholar]
- 26. Lecour S, Andreadou I, Bøtker HE, Davidson SM, Heusch G, Ruiz-Meana M, Schulz R, Zuurbier CJ, Ferdinandy P, Hausenloy DJ, on behalf of the European Union-CARDIOPROTECTION COST ACTION CA16225. IMproving Preclinical Assessment of Cardioprotective Therapies (IMPACT) criteria: guidelines of the EU-CARDIOPROTECTION COST action. Basic Res Cardiol 2021;116:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giricz Z, Varga ZV, Baranyai T, Sipos P, Pálóczi K, Kittel Á, Buzás EI, Ferdinandy P. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol 2014;68:75–78. [DOI] [PubMed] [Google Scholar]
- 28. Baranyai T, Nagy CT, Koncsos G, Onódi Z, Károlyi-Szabó M, Makkos A, Varga ZV, Ferdinandy P, Giricz Z. Acute hyperglycemia abolishes cardioprotection by remote ischemic perconditioning. Cardiovasc Diabetol 2015;14:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brenner GB, Makkos A, Nagy CT, Onódi Z, Sayour NV, Gergely TG, Kiss B, Görbe A, Sághy É, Zádori ZS, Lázár B, Baranyai T, Varga RS, Husti Z, Varró A, Tóthfalusi L, Schulz R, Baczkó I, Giricz Z, Ferdinandy P. Hidden cardiotoxicity of rofecoxib can be revealed in experimental models of ischemia/reperfusion. Cells 2020;9:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weber BY, Brenner GB, Kiss B, Gergely TG, Sayour NV, Tian H, Makkos A, Görbe A, Ferdinandy P, Giricz Z. Rosiglitazone does not show major hidden cardiotoxicity in models of ischemia/reperfusion but abolishes ischemic preconditioning-induced antiarrhythmic effects in rats in vivo. Pharmaceuticals (Basel) 2022;15:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heinen A, Huhn R, Smeele KMA, Zuurbier CJ, Schlack W, Preckel B, Weber NC, Hollmann MW. Helium-induced preconditioning in young and old rat heart: impact of mitochondrial Ca(2+)-sensitive potassium channel activation. Anesthesiology 2008;109:830–836. [DOI] [PubMed] [Google Scholar]
- 32. Zuurbier CJ, Heinen A, Koeman A, Stuifbergen R, Hakvoort TBM, Weber NC, Hollmann MW. Cardioprotective efficacy depends critically on pharmacological dose, duration of ischaemia, health status of animals and choice of anaesthetic regimen: a case study with folic acid. J Transl Med 2014;12:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huhn R, Heinen A, Weber NC, Kerindongo RP, Oei GTML, Hollmann MW, Schlack W, Preckel B. Helium-induced early preconditioning and postconditioning are abolished in obese Zucker rats in vivo. J Pharmacol Exp Ther 2009;329:600–607. [DOI] [PubMed] [Google Scholar]
- 34. Huhn R, Heinen A, Weber NC, Schlack W, Preckel B, Hollmann MW. Ischaemic and morphine-induced post-conditioning: impact of mK(Ca) channels. Br J Anaesth 2010;105:589–595. [DOI] [PubMed] [Google Scholar]
- 35. Huhn R, Weber NC, Preckel B, Schlack W, Bauer I, Hollmann MW, Heinen A. Age-related loss of cardiac preconditioning: impact of protein kinase A. Exp Gerontol 2012;47:116–121. [DOI] [PubMed] [Google Scholar]
- 36. Curtis MJ, Walker MJA. Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc Res 1988;22:656–665. [DOI] [PubMed] [Google Scholar]
- 37. Curtis MJ, Hancox JC, Farkas A, Wainwright CL, Stables CL, Saint DA, Clements-Jewery H, Lambiase PD, Billman GE, Janse MJ, Pugsley MK, Ng GA, Roden DM, Camm AJ, Walker MJA. The lambeth conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol Ther 2013;139:213–248. [DOI] [PubMed] [Google Scholar]
- 38. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. du Sert NP, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Hurst V, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. Reporting animal research: explanation and elaboration for the arrive guidelines 2.0. PLoS Biol 2020; 18:e3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sachdeva J, Dai W, Gerczuk PZ, Kloner RA. Combined remote perconditioning and postconditioning failed to attenuate infarct size and contractile dysfunction in a rat model of coronary artery occlusion. J Cardiovasc Pharmacol Ther 2014;19:567–573. [DOI] [PubMed] [Google Scholar]
- 41. Lassen TR, Hjortbak MV, Hauerslev M, Tonnesen PT, Kristiansen SB, Jensen RV, Bøtker HE. Influence of strain, age, origin, and anesthesia on the cardioprotective efficacy by local and remote ischemic conditioning in an ex vivo rat model. Physiol Rep 2021;9:e14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bromage DI, Pickard JMJ, Rossello X, Ziff OJ, Burke N, Yellon DM, Davidson SM. Remote ischaemic conditioning reduces infarct size in animal in vivo models of ischaemia-reperfusion injury: a systematic review and meta-analysis. Cardiovasc Res 2017;113:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoenig JM, Heisey DM. The abuse of power: the pervasive fallacy of power calculations for data analysis. Am Stat 2001;55:19–24. [Google Scholar]
- 44. Zhang Y, Hedo R, Rivera A, Rull R, Richardson S, Tu XM. Post hoc power analysis: is it an informative and meaningful analysis? Gen Psychiatry 2019;32:e100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lecour S, Du Pré BC, Bøtker HE, Brundel BJJM, Daiber A, Davidson SM, Ferdinandy P, Girao H, Gollmann-Tepeköylü C, Gyöngyösi M, Hausenloy DJ, Madonna R, Marber M, Perrino C, Pesce M, Schulz R, Sluijter JPG, Steffens S, Van Linthout S, Young ME, Van Laake LW. Circadian rhythms in ischaemic heart disease: key aspects for preclinical and translational research: position paper of the ESC working group on cellular biology of the heart. Cardiovasc Res 2022;118:2566–2581. [DOI] [PubMed] [Google Scholar]
- 46. Du PB, Van Veen T, Crnko S, Vos M, Deddens J, Doevendans P, Van Laake L. Variation within variation: comparison of 24-h rhythm in rodent infarct size between ischemia reperfusion and permanent ligation. Int J Mol Sci 2017;18:1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Korevaar DA, Hooft L, ter Riet G. Systematic reviews and meta-analyses of preclinical studies: publication bias in laboratory animal experiments. Lab Anim 2011;45:225–230. [DOI] [PubMed] [Google Scholar]
- 48. Sena ES, Bart van der Worp H, Bath PMW, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol 2010;8:e1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rockwell S, Kimler BF, Moulder JE. Publishing negative results: the problem of publication bias. Radiat Res 2006;165:623–625. [DOI] [PubMed] [Google Scholar]
- 50. Cuijpers P, Smit F, Bohlmeijer E, Hollon SD, Andersson G. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry 2010;196:173–178. [DOI] [PubMed] [Google Scholar]
- 51. Basalay M, Barsukevich V, Mastitskaya S, Mrochek A, Pernow J, Sjöquist PO, Ackland GL, Gourine AV, Gourine A. Remote ischaemic pre- and delayed postconditioning—similar degree of cardioprotection but distinct mechanisms. Exp Physiol 2012;97:908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.