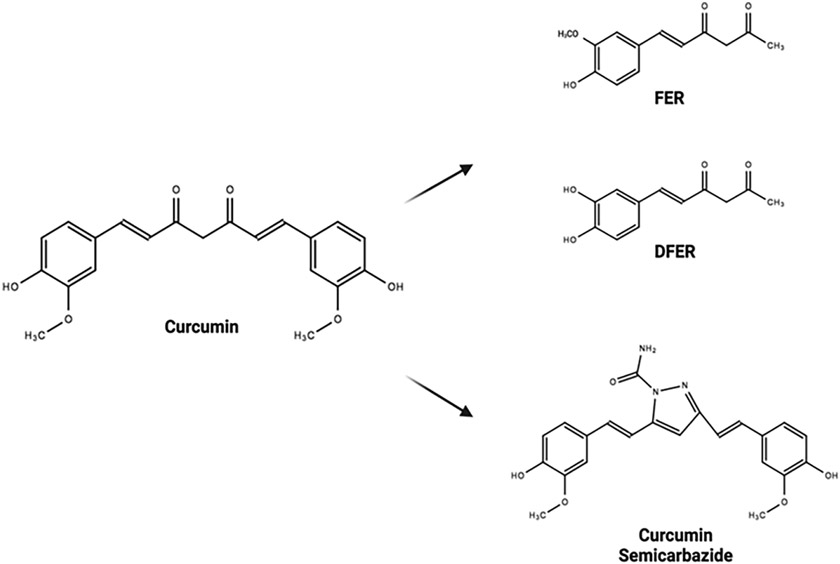
FIG. 2:
Various structural derivatives of CUR have been developed and have shown improvements over base CUR in CRC. Feruloylacetone (FER)/demethoxy-feruloylacetone (DFER), natural degradants of CUR, showed increased apoptosis in CRC cell lines.40 CUR semicarbazide was the most viable synthetic analog created with the purpose of stabilizing the base CUR structure.39 Both variations are examples of modifications on CUR’s chemical structure leading to a drug with a more favorable drug profile (chemical structures were drawn in ChemSpider; image was created with https://biorender.com/).