Abstract
Background
Visceral obesity is associated with high cardiovascular events risk in type 2 diabetes mellitus (T2DM). Whether normal-weight visceral obesity will pose a higher atherosclerotic cardiovascular disease (ASCVD) risk than body mass index (BMI)-defined overweight or obese counterparts with or without visceral obesity remains unclear. We aimed to explore the relationship between general obesity and visceral obesity and 10-year ASCVD risk in patients with T2DM.
Methods
Patients with T2DM (6997) who satisfied the requirements for inclusion were enrolled. Patients were considered to have normal weight when 18.5 kg/m2 ≤ BMI < 24 kg/m2; overweight when 24 kg/m2 ≤ BMI < 28 kg/m2; and obesity when BMI ≥ 28 kg/m2. Visceral obesity was defined as a visceral fat area (VFA) ≥ 100 cm2. Patients were separated into six groups based on BMI and VFA. The odd ratios (OR) for a high 10-year ASCVD risk for different combinations of BMI and VFA were analysed using stepwise logistic regression. Receiver operating characteristic (ROC) curves for diagnosing the high 10-year ASCVD risk were constructed, and areas under the ROC curves were estimated. Potential non-linear relationships between VFA levels and high 10-year ASCVD risk were examined using restricted cubic splines (knot = 4). Multilinear regression was used to identify factors affecting VFA in patients with T2DM.
Results
In patients with T2DM, subjects with normal-weight visceral obesity had the highest 10-year ASCVD risk among the six groups, which had more than a 2-fold or 3-fold higher OR than those who were overweight or obese according to BMI but did not have visceral obesity (all P < 0.05). The VFA threshold for high 10-year ASCVD risk was 90 cm2. Multilinear regression showed significant differences in the effect of age, hypertension, drinking, fasting serum insulin, fasting plasma glucose, 2 h postprandial C-peptide, triglyceride, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol on VFA in patients with T2DM (all P < 0.05).
Conclusions
T2DM patients with normal-weight visceral obesity had a higher 10-year ASCVD risk than BMI-defined overweight or obese counterparts with or without visceral obesity, which should initiate standardised management for ASCVD primary prevention.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-023-01876-7.
Keywords: Normal weight, Visceral obesity, Obesity paradox, Atherosclerotic cardiovascular disease risk, Type 2 diabetes mellitus, Multicentre study
Introduction
Recently, the obesity paradox (OP) has received much attention, especially in cardiovascular disease (CVD), which suggests that patients with different forms of CVD may have an improved outcome if they are overweight or obese (defined by body mass index [BMI]) although they have many more CVD risk factors [1]. This phenomenon was initially described by Gruberg et al. in patients undergoing percutaneous coronary intervention and most likely applied to patients who are overweight and with class I obesity [2]. Subsequent studies have shown a protective role of obesity against overall and cardiovascular mortality in patients with atrial fibrillation, cardiac failure, and coronary artery disease [3]. Possible mechanisms may be related to follow-up, age, BMI, and reverse causation, such as smoking, collider stratification bias, and cardiorespiratory fitness.
Whether OP occurs in patients with type 2 diabetes mellitus (T2DM) remains controversial. Some studies have suggested that individuals who are overweight or obese and have diabetes have lower rates of mortality than those who have normal weight. However, these studies have a number of restrictions owing to their retrospective design and several confounding factors, including smoking habits, antidiabetic treatments, associated pathologies, and lack of data on body fat distribution [4]. Among these, fat distribution, rather than overall adiposity, has attracted much attention. Although BMI is the most commonly used estimator of obesity, it does not capture the distribution of body fat [5]. Coutinho et al. found that visceral obesity was more strongly associated with cardiovascular mortality than BMI [6]. Additionally, compared to peripheral or gluteal-femoral obesity, abdominal fat accumulation is significantly more favourable to coronary disease. Significant CVD risk differences were also detected between visceral and subcutaneous adipose tissue [7, 8]. A prospective study revealed that in Chinese populations with T2DM, all abdominal obesity indexes, including waistline, lipid accumulation product, visceral adiposity index, and Chinese visceral adiposity index, were linked to an elevated risk of CVD events [9].
Normal BMI with increased visceral fat accumulation is relatively common in East Asian populations. Whether normal-weight visceral obesity promotes an increased likelihood of atherosclerotic cardiovascular disease (ASCVD) in patients with T2DM compared to those who have overweight or obesity remains unclear. In this study, we aimed to explore the relationship between general obesity (expressed by BMI) and visceral obesity (expressed by visceral fat area [VFA]) with the 10-year ASCVD risk in T2DM patients without a history of ASCVD, to identify a risk group for early intervention.
Methods
Study design and patients
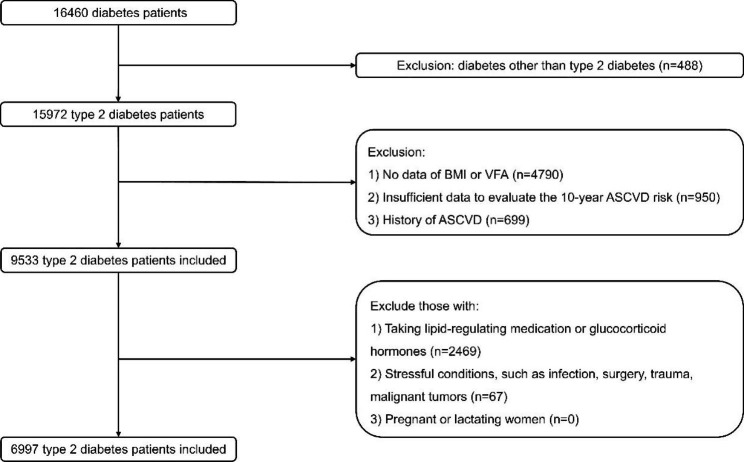
This was a multicentre retrospective observational study. From April 2020 to April 2022, 16,460 adults from 11 National Metabolic Management Centers (MMC) across East China participated in an observational study of ASCVD risk in T2DM (NCT04866667). All participants underwent the same oral questionnaire interviews, systematic physical examinations, blood sample collection, and abdominal fat area measurements. The exclusion criteria were the following: (1) diabetes other than T2DM (n = 488); (2) missing data of BMI or VFA (n = 4790); (3) missing data required to evaluate the 10-year ASCVD risk (n = 950); (4) history of ASCVD (n = 699); (5) use of lipid-regulating medication or glucocorticoid hormones (n = 2469); and (6) presence of stressful conditions (infection, surgery, trauma, malignant tumours, n = 67). Subsequently, 6997 participants were included for the main analysis (Fig. 1). Participants were considered to have normal weight when 18.5 kg/m2 ≤ BMI < 24 kg/m2; overweight when 24 kg/m2 ≤ BMI < 28 kg/m2; and obesity when BMI ≥ 28 kg/m2 [10]. Visceral obesity was defined as a VFA ≥ 100 cm2 [11]. They were separated into six groups based on different combinations of BMI and VFA.
Fig. 1.
Flow chart of patient recruitment
Data collection
We collected data on the patients’ general characteristics through a same questionnaire on the day of patient’ visit. To determine the BMI, weight was divided by height squared.
After an 8 h fasting period, blood samples were taken from the patients on the next morning. All laboratory parameters (biochemistry and diabetes-related) in this study were measured with the same method. The homeostasis model assessment of β-cell function (HOMA-β) was calculated as 20 × serum insulin (FINs)/(plasma glucose [FPG] – 3.5), and the homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as FINs × FPG/22.5.
DUALSCAN HDS-2000 was used to perform a bioelectrical impedance study on the VFA and subcutaneous fat area (SFA) [12]. The evaluation procedure was as follows: (1) on the day preceding the check-up, the patients were instructed to start fasting from 20:00 h; (2) the patients were instructed to lie supine with the wrists, ankles, and abdominal skin exposed while the hand and foot electrode clamps and abdominal electrode belt were installed; and (3) the patients were instructed to hold their breath after breathing calmly, and VFA and SFA were measured at that time.
Definitions
T2DM was diagnosed in accordance with the 2022 American Diabetes Association’s diagnostic criteria of T2DM [13]. Subjects were diagnosed with hypertension if systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg or if they were receiving antihypertensive treatments [14].
ASCVD assessment was based on the 2016 Chinese guidelines for the management of dyslipidaemia in adults, in which the 10-year ASCVD risk was evaluated using the 10-year risk assessment model for coronary heart disease and ischaemic CVD in Chinese adults [15, 16]. The 10-year ASCVD risk was stratified into 21 groups according to low-density lipoprotein cholesterol (LDL-C) or total cholesterol (TC) levels, hypertension, and other ASCVD risk factors. The low risk, medium risk, and high risk of ASCVD is separately defined as < 5%, 5–9% and ≥ 10%. Those meeting any of the following criteria were directly classified as high-risk groups: (1) LDL-C ≥ 4.9 mmol/L (190 mg/dl); (2) 1.8 mmol/L (70 mg/dl) ≤ LDL-C < 4.9 mmol/L (190 mg/dl), and patients with diabetes aged 40 years or older (Table 1).
Table 1.
The assessment process of 10-year ASCVD risk
| Risk factor* (N) |
Stratification of TC (mmol/L) | |||
|---|---|---|---|---|
| 3.1 ≤ TC < 4.1 | 4.1 ≤ TC < 5.2 | 5.2 ≤ TC < 7.2 | ||
| Or 1.8 ≤ LDL-C < 2.6 | Or 2.6 ≤ LDL-C < 3.4 | Or 3.4 ≤ LDL-C < 4.9 | ||
| No hypertension | 0–1 | Low risk (< 5%) | Low risk (< 5%) | Low risk (< 5%) |
| 2 | Low risk (< 5%) | Low risk (< 5%) | Medium risk (5-9%) | |
| 3 | Low risk (< 5%) | Medium risk (5-9%) | Medium risk (5-9%) | |
| Hypertension | 0 | Low risk (< 5%) | Low risk (< 5%) | Low risk (< 5%) |
| 1 | Low risk (< 5%) | Medium risk (5-9%) | Medium risk (5-9%) | |
| 2 | Medium risk (5-9%) | High risk (≥ 10%) | High risk (≥ 10%) | |
| 3 | High risk (≥ 10%) | High risk (≥ 10%) | High risk (≥ 10%) | |
*Risk factors: smoking, low HDL-C and male over 45 years old (female over 55 years old)
Statistical analysis
Data not normally distributed was summarised utilising medians and quartiles, while categorical variables were described as proportions. To identify any distinctions between the six groups, the Kruskal–Wallis rank-sum test was used for multiple samples. The chi-squared test was used to examine discrepancies in categorical variables. The odds ratios (OR) for a high 10-year ASCVD risk for different combinations of BMI and VFA was analysed using stepwise logistic regression. Receiver operating characteristic (ROC) curves for diagnosing the high 10-year ASCVD risk were constructed, and areas under the ROC curves (AUC) were estimated. Potential non-linear relationships between VFA levels and high 10-year ASCVD risk were examined using restricted cubic splines (knot = 4). A heatmap was made to depict the correlation between other variables and VFA in all patients with T2DM. Multilinear regression was used to identify factors affecting VFA in patients with T2DM. SPSS (IBM, version 25.0), MedCalc (version 20.0.4), R (version 3.6), and Prism (GraphPad, version 9.0) were employed for all the analyses. P < 0.05 was deemed statistically significant.
Results
Baseline characteristics
The baseline characteristics of patients with T2DM based on different combinations of BMI and VFA are shown in Table 2. According to the BMI of 6997 patients with T2DM, 2860 (40.9%) had normal weight, 2980 (42.6%) were overweight, and 1157 (16.5%) were obese. Simultaneously, 2777 (39.7%) patients with T2DM were found to have visceral obesity based on the VFA. The results of statistical analysis showed that sex, age, BMI, VFA, SFA, SBP, DBP, hypertension, smoking, drinking, glycosylated haemoglobin A1c (HbA1c), FPG, 2 h postprandial plasma glucose (2 h-PPG), FINs, 2 h postprandial serum insulin (2 h-PINS), fasting C-peptide (FCP), 2 h postprandial C-peptide (2 h-PCP), HOMA-IR, HOMA-β, triglyceride (TG), TC, high-density lipoprotein cholesterol (HDL-C), LDL-C, creatinine (Cr), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma glutamyl-transpeptidase (γ-GT) levels were significantly different among the six groups (all P < 0.05).
Table 2.
Baseline characteristics of the study populations (N = 6997) according to different combinations of BMI and VFA
| Normal Weight | Overweight | Obesity | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic variables | Normal | Visceral obesity | Normal | Visceral obesity | Normal | Visceral obesity | |||||
| Participants | 2531(36.2%) | 329(4.7%) | 1495(21.4%) | 1485(21.2%) | 194(2.8%) | 963(13.8%) | NA | ||||
| Males | 1456(57.5%) | 244(74.2%) | 955(63.9%) | 1041(70.1%) | 111(57.2%) | 621(64.5%) | < 0.001 | ||||
| Age, yr | 55.00(47.00, 62.00) | 57.00(49.00, 62.00) | 55.00(48.00, 61.00) | 55.00(48.00, 63.00) | 54.00(40.00, 62.00) | 55.00(48.00, 62.00) | < 0.001 | ||||
| Body mass index, kg/m2 | 22.13(20.89, 23.13) | 23.09(22.37, 23.55) | 25.39(24.65, 26.23) | 26.12(25.15, 26.98) | 29.08(28.54, 30.74) | 30.13(28.89, 32.52) | < 0.001 | ||||
| Visceral fat area, cm2 | 61.00(44.00, 77.00) | 111.00(104.00, 121.85) | 81.40(69.00, 91.00) | 119.25(108.20, 135.03) | 86.80(78.00, 95.00) | 144.70(122.00, 171.70) | < 0.001 | ||||
| Subcutaneous fat area, cm2 | 122.00(100.13, 145.00) | 151.10(132.00, 171.45) | 168.00(147.00, 193.70) | 185.55(164.00, 210.00) | 229.40(203.63, 278.18) | 252.00(217.00, 301.90) | < 0.001 | ||||
| Systolic blood pressure, mmHg | 123.00(113.00, 136.00) | 129.00(118.50, 144.00) | 129.00(119.00, 141.00) | 130.00(120.00, 141.00) | 133.00(123.00, 141.00) | 135.00(125.00, 147.00) | < 0.001 | ||||
| Diastolic blood pressure, mmHg | 73.00(66.00, 80.00) | 76.00(68.00, 83.00) | 75.00(68.00, 82.00) | 77.00(70.00, 85.00) | 78.00(70.00, 86.00) | 80.00(73.00, 88.00) | < 0.001 | ||||
| Hypertension | 839(33.1%) | 160(48.6%) | 651(43.5%) | 770(51.9%) | 89(45.9%) | 616(64.0%) | < 0.001 | ||||
| Smoking | 727(28.7%) | 133(40.4%) | 473(31.6%) | 581(39.1%) | 34(17.5%) | 312(32.4%) | < 0.001 | ||||
| Drinking | 672(30.9%) | 127(50.4%) | 439(33.3%) | 556(44.5%) | 27(15.6%) | 327(38.9%) | < 0.001 | ||||
| Family history of diabetes | 1086(42.9%) | 142(43.2%) | 625(41.8%) | 626(42.2%) | 82(42.3%) | 384(39.9%) | 0.724 | ||||
| Laboratory variables | |||||||||||
| Glycosylated haemoglobin A1c, mmol/mol | 63.93(47.54, 87.07) | 68.31(51.91, 87.98) | 62.84(48.63, 85.25) | 65.03(51.91, 84.70) | 59.02(45.68, 85.25) | 7.80(61.75, 80.33) | 0.025 | ||||
| Glycosylated haemoglobin A1c, % | 8.00(6.50, 10.30) | 8.40(6.90, 10.20) | 7.90(6.60, 9.95) | 8.10(6.90, 9.90) | 7.55(6.33, 9.95) | 7.80(6.60, 9.50) | 0.025 | ||||
| Fasting blood glucose, mmol/L | 7.71(6.12, 10.31) | 7.95(6.32, 10.28) | 8.00(6.51, 10.65) | 8.16(6.69, 10.52) | 7.47(6.02, 10.14) | 7.85(6.49, 9.84) | < 0.001 | ||||
| 2 h postprandial blood glucose, mmol/L | 15.14(10.94, 19.32) | 15.27(11.57, 19.51) | 15.10(11.21, 18.90) | 14.71(11.32, 18.00) | 13.25(8.88, 17.66) | 14.09(10.81, 17.85) | < 0.001 | ||||
| Fasting insulin, µIU/mL | 5.80(3.10, 10.30) | 6.50(3.70, 10.92) | 8.40(5.10, 14.14) | 8.70(5.76, 13.70) | 13.89(7.47, 29.83) | 11.73(7.07, 19.54) | < 0.001 | ||||
| 2 h postprandial insulin, µIU/mL | 21.97(11.70, 39.78) | 24.44(14.62, 45.13) | 31.24(16.60, 54.94) | 30.61(18.26, 52.88) | 40.10(23.24, 117.99) | 38.70(21.80, 73.80) | < 0.001 | ||||
| Fasting C-peptide, ng/mL | 0.44(0.16, 1.46) | 1.30(0.32, 2.10) | 0.37(0.19, 1.85) | 1.70 (0.29, 2.59) | 0.33(0.22, 1.76) | 1.10(0.27, 2.75) | < 0.001 | ||||
| 2 h postprandial C-peptide, ng/mL | 1.40(0.46, 3.90) | 3.86(0.88, 5.60) | 1.13(0.48, 4.82) | 4.03(0.76, 6.56) | 1.02(0.50, 3.98) | 2.60(0.68, 6.61) | < 0.001 | ||||
| Homeostatic model assessment of insulin resistance | 2.13(1.09, 4.01) | 2.45(1.25, 4.14) | 3.19(1.74, 5.60) | 3.30(2.08, 5.50) | 4.67(2.46, 13.31) | 4.36(2.37, 7.45) | < 0.001 | ||||
| Homeostasis model assessment of β-cell function | 26.07(11.92, 56.39) | 25.42(13.51, 50.69) | 37.07(19.97, 72.95) | 37.15(20.67, 66.18) | 77.47(29.46, 201.27) | 50.82(29.02, 99.94) | < 0.001 | ||||
| Triglyceride, mg/dL | 1.21(0.85, 1.82) | 1.68(1.16, 2.47) | 1.45(1.03, 2.13) | 1.75(1.23, 2.57) | 1.52(1.12, 2.30) | 1.73(1.24, 2.54) | < 0.001 | ||||
| Total cholesterol, mg/dL | 4.72(4.08, 5.42) | 5.06(4.28, 5.70) | 4.79(4.17, 5.50) | 4.89(4.27, 5.72) | 4.89(4.14, 5.61) | 4.91(4.22, 5.64) | < 0.001 | ||||
| High density lipoprotein cholesterol, mg/dL | 1.23(1.03, 1.47) | 1.13(0.97, 1.30) | 1.14(0.97, 1.38) | 1.08(0.92, 1.28) | 1.10(0.95, 1.27) | 1.11(0.95, 1.28) | < 0.001 | ||||
| Low density lipoprotein cholesterol, mg/dL | 2.68(2.16, 3.29) | 2.77(2.28, 3.33) | 2.75(2.22, 3.34) | 2.79(2.26, 3.36) | 3.07(2.44, 3.51) | 2.86(2.22, 3.50) | < 0.001 | ||||
| Serum creatinine, mg/dL | 60.50(51.00, 72.10) | 66.00(56.00, 80.60) | 61.40(51.95, 73.55) | 67.00(56.00, 79.20) | 64.10(50.00, 76.00) | 66.00(54.00, 78.00) | < 0.001 | ||||
| Alanine aminotransferase, IU/L | 19.00(14.00, 29.00) | 25.00(16.00, 41.50) | 23.00(17.00, 35.00) | 27.00(18.00, 41.00) | 30.50(21.00, 50.88) | 31.00(20.00, 50.00) | < 0.001 | ||||
| Aspartate aminotransferase, IU/L | 19.00(15.13, 25.00) | 21.00(17.00, 30.00) | 20.00(16.75, 26.00) | 21.00(16.98, 28.00) | 23.20(19.00, 35.83) | 24.00(18.00, 35.00) | < 0.001 | ||||
| Gamma glutamyl-transpeptidase, IU/L | 22.00(15.00, 36.00) | 33.00(20.00, 60.50) | 26.00(18.00, 43.00) | 34.00(23.00, 55.00) | 34.00(20.02, 56.75) | 37.00(24.00, 61.00) | < 0.001 | ||||
Data are presented as median (IQR) or n (%)
Patients were considered to have normal weight when 18.5 kg/m2 ≤ BMI < 24 kg/m2; overweight when 24 kg/m2 ≤ BMI < 28 kg/m2; and obesity when BMI ≥ 28 kg/m2. Visceral obesity was defined as VFA ≥ 100 cm2
Association between the high 10-year ASCVD risk and BMI/VFA status in the background of T2DM
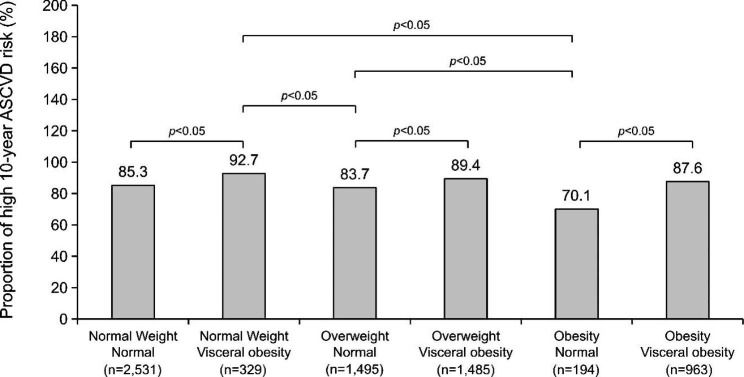
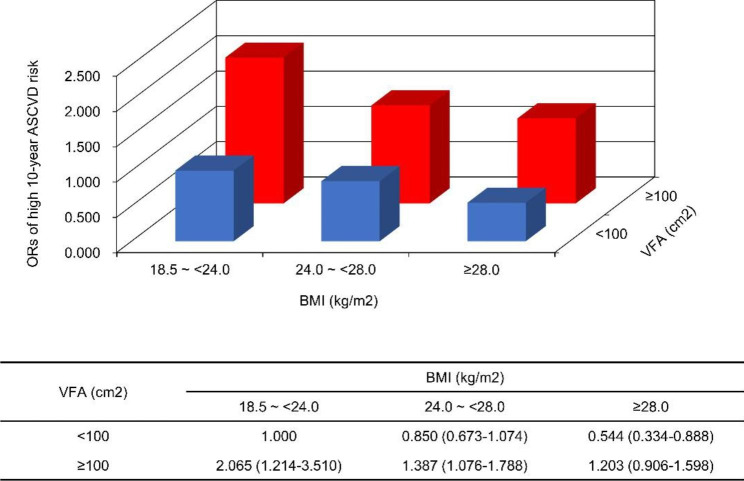
The association between the high 10-year ASCVD risk and BMI/VFA status is shown in Figs. 2 and 3, and 4. In patients with T2DM, subjects with visceral obesity had a higher 10-year ASCVD risk than those with normal visceral fat area, regardless of the BMI category (all P < 0.05). Of note, patients with normal-weight visceral obesity had the highest 10-year ASCVD risk among the six groups, which had more than a 2-fold or 3-fold higher OR than those who were overweight or obese according to BMI but did not have visceral obesity (OR = 2.429, 95% confidence interval [CI]: 1.413–4.175, P = 0.001; OR = 3.792, 95% CI: 1.897–7.579, P < 0.001).
Fig. 2.
The proportion of high 10-year ASCVD risk according to BMI/VFA status in patients with T2DM. Patients were considered to have normal weight when 18.5 kg/m2 ≤ BMI < 24 kg/m2; overweight when 24 kg/m2 ≤ BMI < 28 kg/m2; and obesity when BMI ≥ 28 kg/m2. Visceral obesity was defined as VFA ≥ 100 cm2
Fig. 3.
ORs and 95% CIs for high 10-year ASCVD risk according to BMI/VFA status in T2DM patients. The results were adjusted by age and sex
Fig. 4.
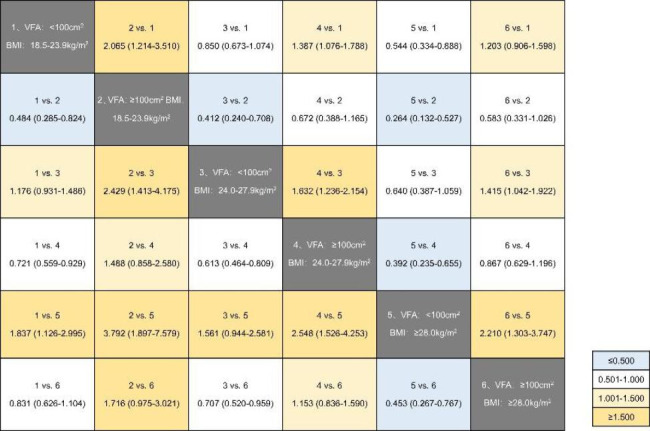
ORs and 95% CIs for high 10-year ASCVD risk according to BMI/VFA status in T2DM patients. The results were adjusted by age and sex
Comparison of BMI, VFA, and BMI + VFA for diagnosing the high 10-year ASCVD risk in patients with T2DM
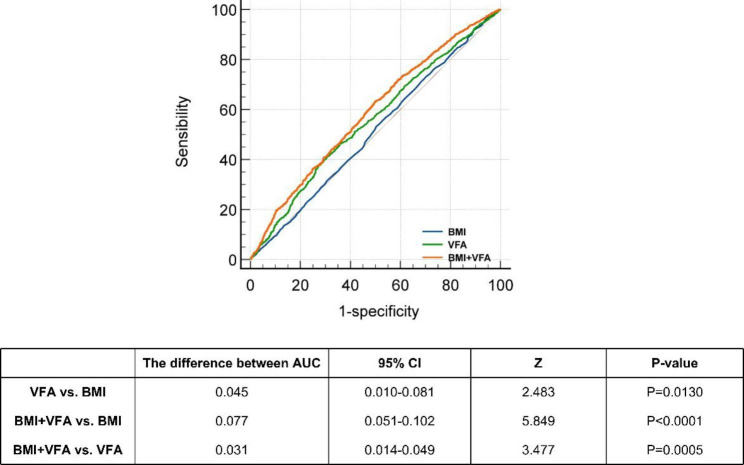
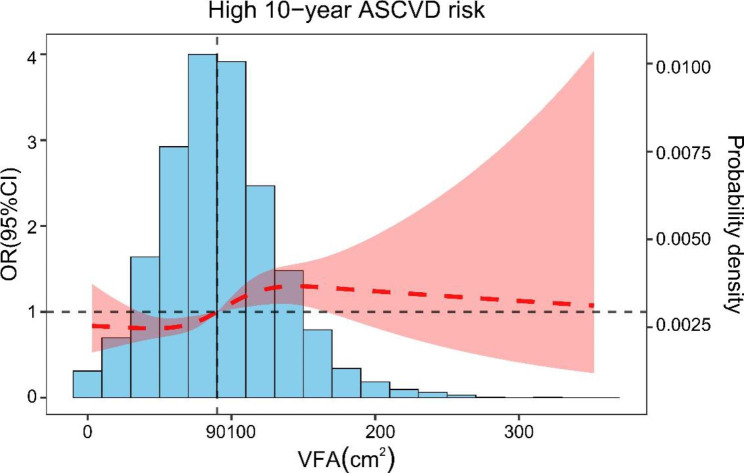
There were 6021 patients with T2DM with (86.1%) high 10-year ASCVD risk and 976 with (13.9%) non-high-risk. The ROC curves and AUCs for BMI, VFA, and BMI + VFA are shown in Fig. 5; Table 3. The AUC of BMI, VFA, and BMI + VFA was respectively 0.511 (95% CI: 0.499–0.523), 0.556 (95% CI: 0.554–0.568), and 0.588 (95% CI: 0.576–0.599). In addition, the difference in AUC between VFA and BMI was 0.045 (Z = 2.483, P = 0.0130), between BMI + VFA and BMI was 0.077 (Z = 5.849, P < 0.0001), and between BMI + VFA and VFA was 0.031 (Z = 3.477, P = 0.0005). The association between VFA and a high 10-year ASCVD risk fitted a non-linear spline model (Fig. 6). When VFA was ≥ 90 cm2, the ORs for high 10-year ASCVD risk was ≥ 1. When VFA was ≥ 100 cm2, the high 10-year ASCVD risk first increased and then decreased.
Fig. 5.
The calculation and comparison of AUCs for diagnosing high 10-year ASCVD risk in T2DM patients. The AUC of BMI, VFA, and BMI + VFA is respectively 0.511 (95% CI: 0.499–0.523), 0.556 (95% CI: 0.554–0.568) and 0.588 (95% CI: 0.576–0.599)
Table 3.
The AUCs of BMI, VFA, and BMI + VFA for diagnosing the high 10-year ASCVD risk in T2DM patients
| AUC | 95% CI | Youden Index | Sensibility | Specificity | |
|---|---|---|---|---|---|
| BMI | 0.511 | 0.499–0.523 | 0.029 | 73.180 | 29.710 |
| VFA | 0.556 | 0.544–0.568 | 0.104 | 41.270 | 69.160 |
| BMI + VFA | 0.588 | 0.576–0.599 | 0.133 | 62.780 | 50.510 |
Fig. 6.
The association between VFA and a high 10-year ASCVD risk in T2DM patients. The result was adjusted by age and sex
Association between the other variables and VFA in patients with T2DM
The correlation analysis of all patients with T2DM revealed that VFA was positively correlated with sex, age, hypertension, smoking, drinking, FINs, 2 h-PINs, FCP, 2 h-PCP, TG, TC and LDL-C and negatively correlated with HDL-C in Spearman’s analyses (all, P < 0.05; Fig. 7). Multilinear regression analysis showed significant differences in the effect of age (B = 0.202, t = 3.837, P < 0.001), hypertension (B = 17.070, t = 14.918, P < 0.001), drinking (B = 9.812, t = 7.491, P < 0.001), FINs (B = 0.057, t = 2.456, P = 0.014), FCP (B = 5.838, t = 14.278, P < 0.001), 2 h-PCP (B = 0.284, t = 2.424, P = 0.015), TG (B = 3.716, t = 9.144, P < 0.001), TC (B = 1.531, t = 2.147, P = 0.032), HDL-C (B = -9.469, t = -6.141, P < 0.001), and LDL-C (B = 2.513, t = 2.955, P = 0.003) on VFA in patients with T2DM (Table 4).
Fig. 7.
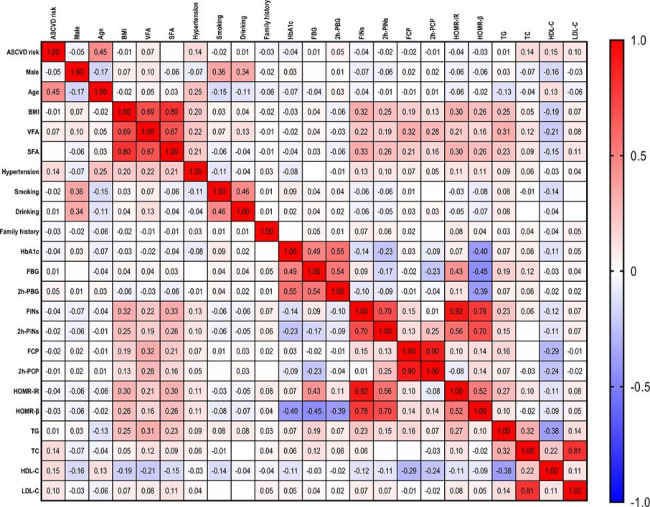
The heatmap depicts the correlational relationships between the other variables and VFA in T2DM patients
Table 4.
A multilinear regression model of VFA in T2DM patients
| Multilinear Regression | R | 0.446 | R2 | 0.199 | Adjusted R2 | 0.197 | Tolerance | VIF |
|---|---|---|---|---|---|---|---|---|
| Variables | B | SE | β | t | Sig. | |||
| Constant | 49.009 | 4.220 | 11.614 | < 0.001 | ||||
| Male | 1.345 | 1.227 | 0.015 | 1.096 | 0.273 | 0.857 | 1.167 | |
| Age | 0.202 | 0.053 | 0.052 | 3.837 | < 0.001 | 0.916 | 1.091 | |
| Hypertension | 17.070 | 1.144 | 0.200 | 14.918 | < 0.001 | 0.936 | 1.069 | |
| Smoking | 1.467 | 1.359 | 0.016 | 1.080 | 0.280 | 0.771 | 1.298 | |
| Drinking | 9.812 | 1.310 | 0.110 | 7.491 | < 0.001 | 0.776 | 1.289 | |
| Fasting insulin | 0.057 | 0.023 | 0.038 | 2.456 | 0.014 | 0.707 | 1.414 | |
| 2 h postprandial insulin | 0.009 | 0.007 | 0.021 | 1.323 | 0.186 | 0.697 | 1.434 | |
| Fasting C-peptide | 5.838 | 0.409 | 0.218 | 14.278 | < 0.001 | 0.722 | 1.385 | |
| 2 h postprandial C-peptide | 0.284 | 0.117 | 0.036 | 2.424 | 0.015 | 0.748 | 1.337 | |
| Triglyceride | 3.716 | 0.406 | 0.144 | 9.144 | < 0.001 | 0.685 | 1.460 | |
| Total cholesterol | 1.531 | 0.713 | 0.044 | 2.147 | 0.032 | 0.396 | 2.527 | |
| High density lipoprotein cholesterol | -9.469 | 1.542 | -0.090 | -6.141 | < 0.001 | 0.789 | 1.267 | |
| Low density lipoprotein cholesterol | 2.513 | 0.850 | 0.056 | 2.955 | 0.003 | 0.463 | 2.160 |
Abbreviations: SE, stand error; Sig, significance. VIF, variance inflation factor: an indicator for diagnosis of collinearity
Discussion
Our analyses of data from 11 MMCs across East China showed that patients with T2DM with normal-weight visceral obesity had a higher 10-year ASCVD risk than those with BMI-defined overweight or obesity, with or without visceral obesity. In addition, VFA was more suggestive of ASCVD risk than BMI, and the threshold for a high 10-year ASCVD risk was 90 cm2. Moreover, we discovered that age, hypertension, drinking, FINs, FCP, 2 h-PCP, TG, TC, HDL-C, and LDL-C levels influenced VFA. Our findings suggest that T2DM patients with normal-weight visceral obesity should initiate standardised management for the primary prevention of ASCVD.
To our knowledge, this is the first study to show that normal-weight visceral obesity, as measured by a combination of BMI and VFA, is associated with a higher 10-year ASCVD risk in patients with T2DM. This warns us that when assessing the ASCVD risk in patients with T2DM, we should pay special attention to those with normal-weight visceral obesity. In addition, it was suggested that conventional and single screening by BMI was no longer adequate for the accurate assessment of obesity, whereas VFA was more suggestive. Furthermore, the threshold for VFA suggestive of a high 10-year ASCVD risk was 90 cm2. Previous studies have linked elevated VFA levels to an elevated risk of CVD in individuals with T2DM [17, 18]. Summarising the results of several cardiometabolic imaging studies, Piche et al. [19] found that some normal weight or overweight people with excessive visceral adipose tissue were at high risk, which is usually accompanied by fat build-up in normal lean tissue. Excess ectopic fat and visceral adipose tissue significantly determine the risk of CVD [20, 21], while subcutaneous adipose tissue is linked to retained insulin sensitivity and reduces the risk of metabolic diseases [22–24]. Thus, BMI and VFA should be coupled for the assessment of obesity.
We attempted to explore the factors associated to VFA. After correcting for sex and age, we found that hypertension; the degree of alcohol consumption; and the levels of FINs, FCP, 2 h-PCP, TG, TC, and LDL-C were positively correlated to the levels of VFA. The lower the HDL-C levels, the higher the VFA levels. Age is not considered as an independent criterion in current adult obesity assessment guidelines [25]. However, age-related body composition changes include visceral fat, reduced bone mineral density, and sarcopenia [26]. Results from previous research regarding the connection between alcohol consumption and overweight/obesity are inconsistent. A recent study that analysed 127 large cohorts found that when comparing between light alcohol drinkers or non-alcohol drinkers, heavy alcohol drinkers had a higher risk of developing abdominal obesity [27], which is consistent with our findings. Several epidemiological investigations have indicated that accumulation of VFA is linked with the risk of hypertension, insulin resistance, and dyslipidaemia [28–30]. Our research found that insulin resistance was related to visceral obesity. Kolb et al. [31, 32] have provided support for the obesity-promoting role of insulin. Dyslipidaemia, which is characterised by increased LDL-C and TG levels and reduced HDL-C levels, has been established as a characteristic of cardiovascular and obesity diseases [33, 34]. Zhu et al. [35] found that participants with central obesity residing in Shanghai Suburban had higher levels of TG, TC, and LDL-C and lower levels of HDL-C, which is consistent with our results. Therefore, controlling blood pressure, reducing alcohol intake, and treating insulin resistance and dyslipidaemia early in these patients can reduce obesity and CVD risk.
Visceral obesity can be assessed using various methods including computed tomography (CT), magnetic resonance imaging (MRI), bioelectrical impedance analysis, and anthropometric indicators. CT is the gold standard for measuring visceral adipose tissue, which can be done quickly and analysed using a commercial software. However, it involves exposure to radiation; therefore, it is not suitable for continuous assessment over time or for the assessment of changes after an intervention. MRI does not involve radiation and can be used for continuous assessment over time; however, is time-consuming and expensive [36, 37]. Anthropometric indicators are easy to measure but do not correlate well with direct imaging-based assessments of visceral obesity. CT and MRI provide greater sensitivity and specificity for measuring VFA [37]. In our study we employed, bioelectrical impedance analysis, which is a non-invasive, convenient, and accurate method. Omura-Ohata et al. [38] revealed that bioelectrical impedance analysis could be an alternative to CT as a non-intrusive and inexpensive method for assessing VFA in patients with diabetes. Moreover, Park et al. [39] found that dual abdominal bioelectrical impedance analysis performed on a DUALSCAN HDS-2000 machine was more precise in determining abdominal VFA than whole-body bioelectrical impedance analysis referenced to CT.
Over the last few decades, several widely recognised algorithms and models for assessing ASCVD risk have been created and revised in the USA and Europe; one of the most widely used is the Pooled Cohort Equations (PCEs) of the 2013 American College of Cardiology/American Heart Association assessment of cardiovascular risk guidelines [40]. However, this does not perform well in East Asian populations [41, 42]. Recent research has shown that the ischaemic CVD risk indicated by the Chinese model is lower than the 10-year ASCVD risk predicted by PCEs [43, 44]. The 10-year risk assessment model for coronary heart disease and ischaemic CVD in Chinese adults, combined with the spectrum of diseases and prevalence of cardiovascular risk factors in China, has been widely used in a large number of studies [45, 46]. Therefore, we chose the Chinese model to evaluate the 10-year ASCVD risk in the patients in our study [16].
This study had several limitations. First, owing to the study’s cross-sectional design, we were unable to investigate the long-term dynamic connection between obesity status and ASCVD risk. Second, we could not assess the impact of therapeutic interventions, such as lifestyle changes and medications, on the risk of ASCVD in individuals with normal-weight visceral obesity. However, the long-term follow-up of the patients included in our study is ongoing.
Conclusion
Our study found that T2DM patients with normal-weight visceral obesity had a higher 10-year ASCVD risk than individuals with T2DM and BMI-defined overweight or obesity, with or without visceral obesity, which should initiate standardised management for ASCVD primary prevention. This may have significant clinical implications because most people do not consider patients with T2DM with normal BMI and visceral obesity as a priority population for ASCVD primary prevention. Future research should focus on identifying factors associated with the development of normal-weight visceral obesity and enhancing our understanding in the impact of normal-weight visceral obesity on health outcomes. In this regard, the use of a combined BMI and VFA measure may provide a better stratification of obesity-related risk factors in clinical practice than relying solely on either method alone.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1: Figure S1 The proportion of high 10-year ASCVD risk according to BMI/VFA status in male patients with T2DM. Figure S2. The proportion of high 10-year ASCVD risk according to BMI/VFA status in female patients with T2DM. Table S1. Association between the other variables and VFA in T2DM patients. Table S2. Linear regression analysis of VFA in T2DM patients
Acknowledgements
The authors gratefully acknowledge the participation and cooperation of all patients in this study, as well as that of the personnel from the Department of Endocrinology of the Zhejiang Provincial People’s Hospital, Fenghua District Traditional Chinese Medicine Hospital of Ningbo, Yuhuan Second People’s Hospital, Ningbo First Hospital, Shaoxing Shangyu People’s Hospital, Lishui People’s Hospital, the People’s Hospital of Putuo Zhoushan, Yueqing People’s Hospital, Shaoxing People’s Hospital, Xianju people’s hospital, and the First Hospital of Jiaxing. Moreover, the authors also express their gratitude for the support of the Key Laboratory of Endocrine Gland Diseases in Zhejiang Province.
Abbreviations
- OP
Obesity paradox
- CVD
Cardiovascular disease
- BMI
Body mass index
- T2DM
Type 2 diabetes mellitus
- ASCVD
Atherosclerotic cardiovascular disease
- VFA
Visceral fat area
- MMC
Metabolic Management Centre
- HOMA-β
Homeostasis model assessment of β-cell function
- FINS
Fasting serum insulin
- FPG
Fasting plasma glucose
- HOMA-IR
Homeostasis model assessment of insulin resistance
- SFA
Subcutaneous fat area
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- LDL-C
Low-density lipoprotein cholesterol
- TC
Total cholesterol
- OR
Odd ratio
- ROC
Receiver operating characteristic
- AUC
Area under the ROC curve
- HbA1c
Glycosylated haemoglobin A1c
- 2h-PPG
2 h postprandial plasma glucose
- 2h-PINS
2 h postprandial serum insulin
- FCP
Fasting C-peptide
- 2h-PCP
2 h postprandial C-peptide
- TG
Triglyceride
- HDL-C
High-density lipoprotein cholesterol
- Cr
Creatinine
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- γ-GT
Gamma glutamyl-transpeptidase
- CI
Confidence interval
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- PCEs
Pooled Cohort Equations
Authors’ contributions
JZ, YH and HX analyzed the data and wrote the paper. JZ, YL and XW contributed to study conceptualization. JZ, YH, HX, YL, JZ, QZ, LL, WT, RC, QG, XZ, QY, ZX, QZ and XW were responsible for the acquisition, analysis, or interpretation of data. All the authors have approved the final version of article and were involved in the decision to submit the manuscript for publication.
Funding
This work was supported by the National Natural Science Foundation of China (81970714), the Joint Funds of the Zhejiang Provincial Natural Science Foundation of China under Grant No. LHDMZ23H070001, Science and technology innovation leading talent project of Zhejiang ten thousand people plan (2021R52022) and Zhejiang province health innovative talents project (2021-CXRC07-01).
Data Availability
The data from this study are available from the corresponding author upon reasonable request. The authors declare that they have no conflict of interest.
Declarations
Ethics approval and consent to participate
This research was carried out in conformity with the principles of the Helsinki Declaration, which was approved by the Ethics Committee of Zhejiang province people’s hospital, and each participants gave informed written consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, et al. An overview and update on obesity and the obesity Paradox in Cardiovascular Diseases. Prog Cardiovasc Dis. 2018;61(2):142–50. doi: 10.1016/j.pcad.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39(4):578–84. doi: 10.1016/S0735-1097(01)01802-2. [DOI] [PubMed] [Google Scholar]
- 3.Vecchie A, Dallegri F, Carbone F, Bonaventura A, Liberale L, Portincasa P, et al. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med. 2018;48:6–17. doi: 10.1016/j.ejim.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Gravina G, Ferrari F, Nebbiai G. The obesity paradox and diabetes. Eat Weight Disord. 2021;26(4):1057–68. doi: 10.1007/s40519-020-01015-1. [DOI] [PubMed] [Google Scholar]
- 5.Parra-Soto S, Petermann-Rocha F, Boonpor J, Gray SR, Pell JP, Celis-Morales C, et al. Combined association of general and central obesity with incidence and mortality of cancers in 22 sites. Am J Clin Nutr. 2021;113(2):401–9. doi: 10.1093/ajcn/nqaa335. [DOI] [PubMed] [Google Scholar]
- 6.Coutinho T, Goel K, Correa de Sa D, Carter RE, Hodge DO, Kragelund C, et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of “normal weight central obesity. J Am Coll Cardiol. 2013;61(5):553–60. doi: 10.1016/j.jacc.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Kwon HW, Lee SM, Lee JW, Oh JE, Lee SW, Kim SY. Association between volume and glucose metabolism of abdominal adipose tissue in healthy population. Obes Res Clin Pract. 2017;11(5 Suppl 1):133–43. doi: 10.1016/j.orcp.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Mongraw-Chaffin M, Allison MA, Burke GL, Criqui MH, Matsushita K, Ouyang P, et al. CT-Derived body fat distribution and Incident Cardiovascular Disease: the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2017;102(11):4173–83. doi: 10.1210/jc.2017-01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiao T, Luo T, Pei H, Yimingniyazi B, Aili D, Aimudula A, et al. Association between abdominal obesity indices and risk of cardiovascular events in chinese populations with type 2 diabetes: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):225. doi: 10.1186/s12933-022-01670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinese Nutrition Society. Obesity P, Control S, Chinese Nutrition Society Clinical. Nutrition S, Chinese Preventive Medicine Association Behavioral Health S. Chinese Preventive Medicine Association. Health S. Expert Consensus on obesity Prevention and Treatment in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2022;43(5):609–26. doi: 10.3760/cma.j.cn112338-20220402-00253. [DOI] [PubMed] [Google Scholar]
- 11.Ryo M, Maeda K, Onda T, Katashima M, Okumiya A, Nishida M, et al. A new simple method for the measurement of visceral fat accumulation by bioelectrical impedance. Diabetes Care. 2005;28(2):451–3. doi: 10.2337/diacare.28.2.451. [DOI] [PubMed] [Google Scholar]
- 12.Enomoto M, Adachi H, Fukami A, Kumagai E, Nakamura S, Nohara Y, et al. A useful Tool as a medical checkup in a General Population-Bioelectrical Impedance Analysis. Front Cardiovasc Med. 2017;4:3. doi: 10.3389/fcvm.2017.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association Professional Practice C Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):17–S38. doi: 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- 14.Joint Committee for Guideline R. 2018 Chinese Guidelines for Prevention and Treatment of Hypertension-A report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatr Cardiol. 2019;16(3):182–241. [DOI] [PMC free article] [PubMed]
- 15.Joint committee issued Chinese guideline for the management of dyslipidemia in a 2016 chinese guideline for the management of dyslipidemia in adults. Zhonghua xin xue guan bing za zhi. 2016;44(10):833–53. doi: 10.3760/cma.j.issn.0253-3758.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Liu X, Li X, Li Y, Zhao L, Chen Z, et al. Estimation of 10-year risk of fatal and nonfatal ischemic cardiovascular diseases in chinese adults. Circulation. 2006;114(21):2217–25. doi: 10.1161/CIRCULATIONAHA.105.607499. [DOI] [PubMed] [Google Scholar]
- 17.Xu L, Song P, Xu J, Zhang H, Yu C, Guan Q, et al. Viscus fat area contributes to the Framingham 10-year general cardiovascular disease risk in patients with type 2 diabetes mellitus. Life Sci. 2019;220:69–75. doi: 10.1016/j.lfs.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Qiu Y, Deng X, Sha Y, Wu X, Zhang P, Chen K, et al. Visceral Fat Area, not subcutaneous Fat Area, is Associated with Cardiac Hemodynamics in Type 2 diabetes. Diabetes Metab Syndr Obes. 2020;13:4413–22. doi: 10.2147/DMSO.S284420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piche ME, Tchernof A, Despres JP. Obesity phenotypes, diabetes, and Cardiovascular Diseases. Circ Res. 2020;126(11):1477–500. doi: 10.1161/CIRCRESAHA.120.316101. [DOI] [PubMed] [Google Scholar]
- 20.Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol. 2007;27(5):996–1003. doi: 10.1161/ATVBAHA.106.131755. [DOI] [PubMed] [Google Scholar]
- 21.Okura T, Nakata Y, Yamabuki K, Tanaka K. Regional body composition changes exhibit opposing effects on coronary heart disease risk factors. Arterioscler Thromb Vasc Biol. 2004;24(5):923–9. doi: 10.1161/01.ATV.0000125702.26272.f6. [DOI] [PubMed] [Google Scholar]
- 22.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among japanese Americans. Diabetes Care. 2000;23(4):465–71. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 23.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27(2):372–7. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 24.Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34(11–12):616–21. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- 25.Bray GA, Heisel WE, Afshin A, Jensen MD, Dietz WH, Long M, et al. The science of obesity management: an endocrine Society Scientific Statement. Endocr Rev. 2018;39(2):79–132. doi: 10.1210/er.2017-00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villareal DT, Apovian CM, Kushner RF, Klein S. American Society for N, Naaso T O S. obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, the obesity society. Obes Res. 2005;13(11):1849–63. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 27.Golzarand M, Salari-Moghaddam A, Mirmiran P. Association between alcohol intake and overweight and obesity: a systematic review and dose-response meta-analysis of 127 observational studies. Crit Rev Food Sci Nutr. 2022;62(29):8078–98. doi: 10.1080/10408398.2021.1925221. [DOI] [PubMed] [Google Scholar]
- 28.Feng Y, Yang X, Li Y, Wu Y, Han M, Qie R, et al. Metabolic score for visceral Fat: a reliable indicator of visceral obesity for predicting risk for hypertension. Nutrition. 2022;93:111443. doi: 10.1016/j.nut.2021.111443. [DOI] [PubMed] [Google Scholar]
- 29.Oikonomou EK, Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol. 2019;16(2):83–99. doi: 10.1038/s41569-018-0097-6. [DOI] [PubMed] [Google Scholar]
- 30.Vekic J, Zeljkovic A, Stefanovic A, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V. Obesity and dyslipidemia. Metabolism. 2019;92:71–81. doi: 10.1016/j.metabol.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Kolb H, Kempf K, Rohling M, Martin S. Insulin: too much of a good thing is bad. BMC Med. 2020;18(1):224. doi: 10.1186/s12916-020-01688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolb H, Stumvoll M, Kramer W, Kempf K, Martin S. Insulin translates unfavourable lifestyle into obesity. BMC Med. 2018;16(1):232. doi: 10.1186/s12916-018-1225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su X, Peng D. The exchangeable apolipoproteins in lipid metabolism and obesity. Clin Chim Acta. 2020;503:128–35. doi: 10.1016/j.cca.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. 2021;18(10):689–700. doi: 10.1038/s41569-021-00541-4. [DOI] [PubMed] [Google Scholar]
- 35.Zhu J, Zhang Y, Wu Y, Xiang Y, Tong X, Yu Y et al. Obesity and dyslipidemia in chinese adults: a cross-sectional study in Shanghai, China. Nutrients. 2022;14(11). [DOI] [PMC free article] [PubMed]
- 36.Neeland IJ, Grundy SM, Li X, Adams-Huet B, Vega GL. Comparison of visceral fat mass measurement by dual-X-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: the Dallas Heart Study. Nutr Diabetes. 2016;6(7):e221. doi: 10.1038/nutd.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neeland IJ, Poirier P, Despres JP. Cardiovascular and metabolic heterogeneity of obesity: Clinical Challenges and Implications for Management. Circulation. 2018;137(13):1391–406. doi: 10.1161/CIRCULATIONAHA.117.029617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omura-Ohata Y, Son C, Makino H, Koezuka R, Tochiya M, Tamanaha T, et al. Efficacy of visceral fat estimation by dual bioelectrical impedance analysis in detecting cardiovascular risk factors in patients with type 2 diabetes. Cardiovasc Diabetol. 2019;18(1):137. doi: 10.1186/s12933-019-0941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park KS, Lee DH, Lee J, Kim YJ, Jung KY, Kim KM, et al. Comparison between two methods of bioelectrical impedance analyses for accuracy in measuring abdominal visceral fat area. J Diabetes Complications. 2016;30(2):343–9. doi: 10.1016/j.jdiacomp.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 40.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 41.Jung KJ, Jang Y, Oh DJ, Oh BH, Lee SH, Park SW, et al. The ACC/AHA 2013 pooled cohort equations compared to a korean risk prediction model for atherosclerotic cardiovascular disease. Atherosclerosis. 2015;242(1):367–75. doi: 10.1016/j.atherosclerosis.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 42.Lee CH, Woo YC, Lam JK, Fong CH, Cheung BM, Lam KS, et al. Validation of the pooled cohort equations in a long-term cohort study of Hong Kong Chinese. J Clin Lipidol. 2015;9(5):640–646e642. doi: 10.1016/j.jacl.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Lin HQ, Wu JY, Chen ML, Chen FQ, Liao YJ, Wu YT, et al. Prevalence of dyslipidemia and prediction of 10-year CVD risk among older adults living in southeast coastal regions in China: a cross-sectional study. Clin Interv Aging. 2019;14:1119–29. doi: 10.2147/CIA.S207665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M, Jiang Y, Wang LM, Li YC, Huang ZJ, Li JH, et al. Prediction of 10-year atherosclerotic Cardiovascular Disease risk among adults aged 40–79 years in China: a nationally Representative Survey. Biomed Environ Sci. 2017;30(4):244–54. doi: 10.3967/bes2017.034. [DOI] [PubMed] [Google Scholar]
- 45.Jafar TH, Tan NC, Shirore RM, Allen JC, Finkelstein EA, Hwang SW, et al. Integration of a multicomponent intervention for hypertension into primary healthcare services in Singapore-A cluster randomized controlled trial. PLoS Med. 2022;19(6):e1004026. doi: 10.1371/journal.pmed.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Liu J, Zhou B, Li X, Wu Z, Meng H, et al. Reducing the 10-year risk of ischemic cardiovascular disease to receive early cardiovascular benefits from bariatric surgery for obesity in China. Front Cardiovasc Med. 2022;9:978682. doi: 10.3389/fcvm.2022.978682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1 The proportion of high 10-year ASCVD risk according to BMI/VFA status in male patients with T2DM. Figure S2. The proportion of high 10-year ASCVD risk according to BMI/VFA status in female patients with T2DM. Table S1. Association between the other variables and VFA in T2DM patients. Table S2. Linear regression analysis of VFA in T2DM patients
Data Availability Statement
The data from this study are available from the corresponding author upon reasonable request. The authors declare that they have no conflict of interest.