Fig. 3. Synthesis of coral terpenes from synthetic GGPP analogues in vitro.
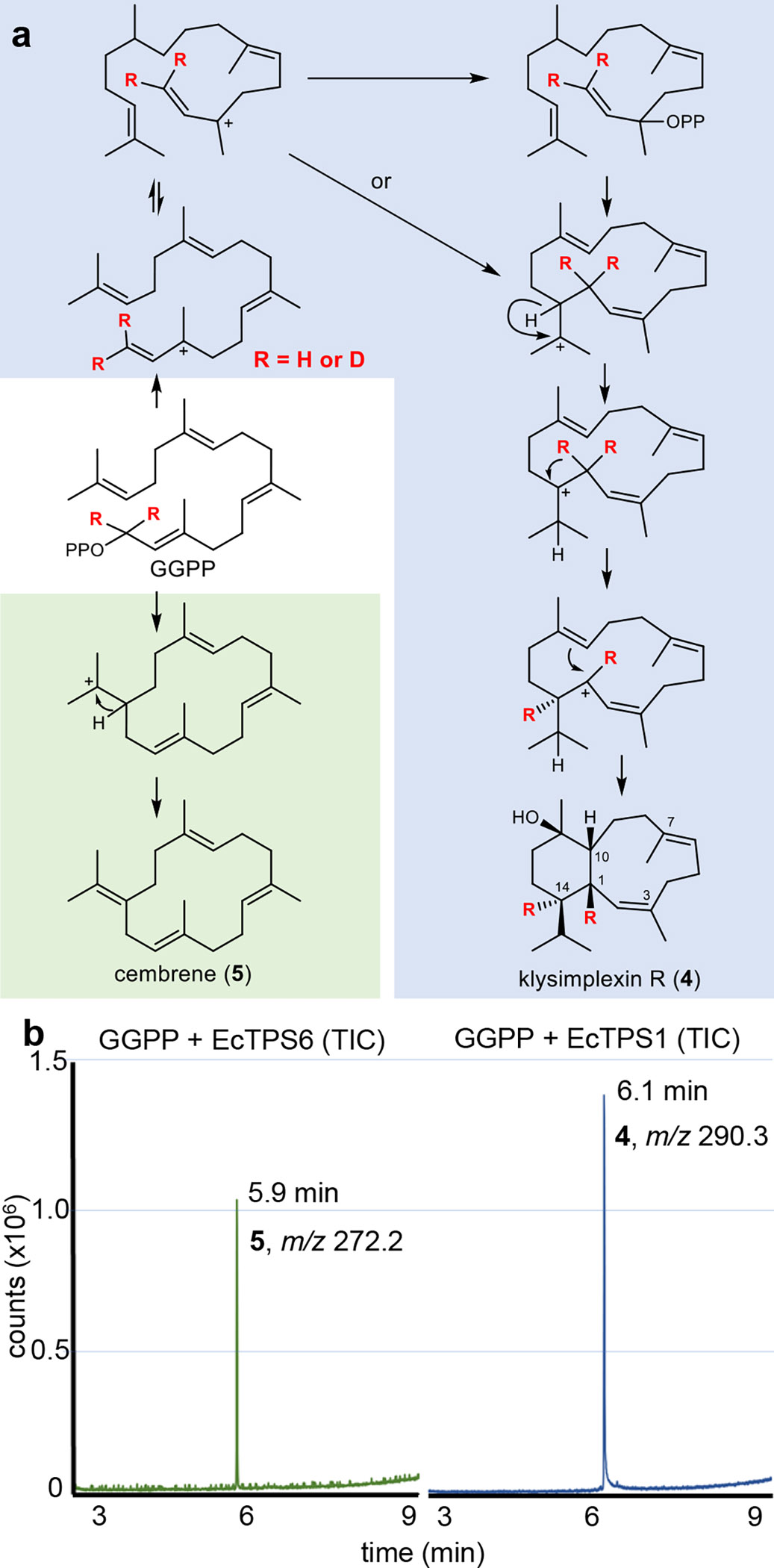
(a) The coral terpene precursors klysimplexin R (4) and cembrene (5) were synthesized by incubating GGPP with purified coral enzymes EcTPS1 and EcTPS6, respectively. EcTPS1 was also treated with deuterated GGPP, resulting in deuterium migration supporting an unprecedented cyclization mechanism (see text for details). (b) GCMS traces (total ion current) from incubation of EcTPS1 (right) and EcTPS6 (left). Analytical scale assays indicated that 4 (tr=6.11 min, m/z = 290.3) and 5 (tr=5.88 min, m/z = 272.2) were the sole cyclized products. The no-enzyme control and other 3 enzyme reactions are shown in Extended Data Fig. 5. The resulting electron impact mass spectra, as well as a full 2D NMR data set, support the structures of the compounds as shown (Supplementary Note 1).
