Abstract
The natural history of radiorecurrent high-risk prostate cancer (HRPCa) is not well-described. To better understand its clinical course, we evaluated rates of distant metastases (DM) and prostate cancer-specific mortality (PCSM) in a cohort of 978 men with radiorecurrent HRPCa who previously received either external beam radiation therapy (EBRT, n = 654, 67%) or EBRT + brachytherapy (EBRT + BT, n = 324, 33%) across 15 institutions from 1997 to 2015. In men who did not die, median follow-up after treatment was 8.9 yr and median follow-up after biochemical recurrence (BCR) was 3.7 yr. Local and systemic therapy salvage, respectively, were delivered to 21 and 390 men after EBRT, and eight and 103 men after EBRT + BT. Overall, 435 men developed DM, and 248 were detected within 1 yr of BCR. Measured from time of recurrence, 5-yr DM rates were 50% and 34% after EBRT and EBRT + BT, respectively. Measured from BCR, 5-yr PCSM rates were 27% and 29%, respectively. Interval to BCR was independently associated with DM (p < 0.001) and PCSM (p < 0.001). These data suggest that radiorecurrent HRPCa has an aggressive natural history and that DM is clinically evident early after BCR. These findings underscore the importance of further investigations into upfront risk assessment and prompt systemic evaluation upon recurrence in HRPCa.
Patient summary:
High-risk prostate cancer that recurs after radiation therapy is an aggressive disease entity and spreads to other parts of the body (metastases). Some 60% of metastases occur within 1 yr. Approximately 30% of these patients die from their prostate cancer.
Keywords: Biochemical recurrence, Brachytherapy boost, EBRT, External beam radiation therapy, High-risk prostate cancer, Prostate cancer, Radiorecurrence, Recurrent prostate cancer
More than 50% of men with high-risk prostate cancer (HRPCa)—defined as the presence of Gleason grade group 4–5 disease, clinical T stage 3–4, or prostate-specific antigen (PSA) >20 ng/ml—will develop biochemical recurrence (BCR) during extended follow-up [1]. These recurrences, termed “radiorecurrence” in the context of BCR after radiotherapy (RT), are currently defined as a rise in PSA of ≥2 ng/ml above the post-RT nadir [2] and could represent local, regional, or distant disease. Management options include observation, androgen deprivation therapy (ADT), metastasis-directed therapy, and local salvage therapies [3,4], but the choice of treatment is obfuscated by a lack of prospective studies and a limited understanding of the natural history of radiorecurrent HRPCa. The purpose of this study was to evaluate clinical outcomes after BCR among patients who received definitive external beam RT (EBRT) or EBRT with a brachytherapy boost (EBRT + BT) for HRPCa.
The study population consisted of 978 men who developed BCR (defined as PSA nadir + ≥2 ng/ml [2]) after receiving EBRT (n = 654, 67%) or EBRT + BT (n = 324, 33%) for HRPCa across 15 institutions from 1997 to 2015 (Supplementary Table 1). Follow-up times from completion of RT and from the date of BCR were summarized using the median and interquartile range (IQR) for men who did not die, and separately for men who did not develop distant metastasis (DM). The primary outcomes were DM and prostate cancer–specific mortality (PCSM), measured from the date of BCR. To better understand whether certain pretreatment and post-treatment characteristics might be associated with the development of metastases or mortality events after recurrence, multivariable Cox proportional-hazard models were used to evaluate the effects of the following prespecified covariates: age, Gleason grade group, ln(initial PSA [iPSA]), clinical T stage, and interval to BCR (calculated from the RT completion date to the date of BCR) on DM and PCSM. Models were assessed for the proportionality hazard assumption based on weighted residuals and observational tools [5]. Multivariable Cox proportional-hazard models adjusted for the same above-mentioned covariates were used to compare differences in time to systemic salvage therapy (initiation of new systemic therapies—including ADT and nonhormonal therapies—for radiorecurrence). Fine-Gray competing-risk regression models were also used to determine covariate-adjusted subdistribution hazard ratios (sHRs; adjusted for age at treatment, ln[iPSA], Gleason grade group, clinical T stage, interval to BCR, and treatment arm) for DM and PCSM after BCR. Death was the competing risk for DM and other-cause mortality was the competing risk for PCSM. Fisher’s exact test and a Wilcoxon rank-sum test were used to evaluate differences in categorical and continuous variables, respectively, between EBRT and EBRT + BT. Tests of heterogeneity for all covariates between the treatment groups for both DM and PCSM were assessed using multivariable Cox regression models that included interaction terms between treatment arm and other predictors. Kaplan-Meier methods were used to generate survival curves.
For men who did not die, median follow-up was 8.9 yr from RT completion and 3.7 yr from BCR. Clinicopathologic features at diagnosis are shown in Supplementary Table 2. Details of the treatments received are shown in Supplementary Table 3. Most men (90%) received neoad-juvant/concurrent ADT (90% with EBRT, median duration 23 mo; 91% with EBRT + BT, median duration 8 mo). ADT duration was significantly longer in the EBRT group (p < 0.001). Local salvage was performed in 21 patients after EBRT and in eight patients after EBRT + BT. Systemic salvage was used in 390 men after EBRT and 103 men after EBRT + BT; there was no significant difference between time to initiation of systemic salvage between EBRT and EBRT + BT (adjusted HR 1.01, 95% confidence interval [CI] 1.00–1.03; p = 0.14).
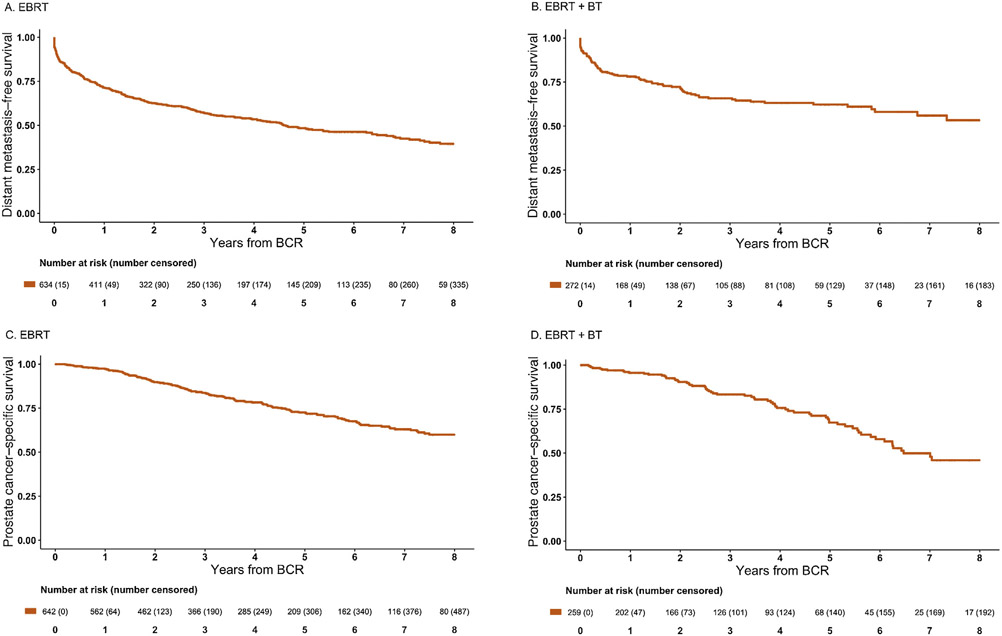
Kaplan-Meier curves for DM-free survival (DMFS) and prostate cancer–specific survival (PCSS) are presented in Figure 1. Measured from time of BCR, the 5-yr cumulative DM incidence rate was 50% among men treated with EBRT and 34% among men treated with EBRT + BT. Development of BCR within 3 yr of RT occurred in 378 men (261 after EBRT and 117 after EBRT + BT). A total of 330 men developed DM after EBRT and 105 developed DM after EBRT + BT. Among 435 men who developed distant failure, DM occurred within 1 yr of BCR in 189 EBRT patients and 59 EBRT + BT patients. Measured from BCR, the 5-yr cumulative PCSM incidence was 27% overall, with incidences of 27% after EBRT and 29% after EBRT + BT.
Fig. 1 –
Kaplan-Meier curves for (A,B) distant metastasis–free survival and (C,D) prostate cancer–specific survival following biochemical recurrence (BCR) in men with high-risk prostate cancer treated initially with external beam radiotherapy (EBRT) or external beam radiotherapy plus brachytherapy boost (EBRT + BT). (A) Distant metastasis–free survival following BCR after EBRT. (B) Distant metastasis–free survival following BCR after EBRT + BT. (C) Prostate cancer–specific survival following BCR after EBRT. (D) Prostate cancer–specific survival following BCR after EBRT + BT.
Several significant interaction terms between treatment arm and predictors in Cox regression models for time to PCSM and DM were identified, suggesting heterogeneity of covariates across treatment arms (Supplementary Table 4). Therefore, Cox models for DM and PCSM are presented for each treatment arm (EBRT and EBRT + BT) separately in Table 1, with competing-risk regression models in Supplementary Table 5. On stratified multivariable Cox regression for both EBRT and EBRT + BT, interval to BCR was a predictor for DM (EBRT: HR 0.84, 95% CI 0.78–0.89; p < 0.001; EBRT + BT: HR 0.87, 95% CI 0.77–0.99; p = 0.03) and PCSM (EBRT: HR 0.72, 95% CI 0.63–0.81; p < 0.001; EBRT + BT: HR 0.81, 95% CI 0.69–0.94; p = 0.008; Table 1).
Table 1 –
Multivariable Cox proportional-hazard model for prediction of distant metastasis and prostate cancer–specific mortality stratified by treatment for men with radiorecurrent high-risk prostate cancer
| Distant metastasis |
Prostate cancer–specific mortality |
|||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| External beam radiation therapy | ||||
| Interval to biochemical failure a | 0.84 (0.78–0.89) | <0.001 | 0.72 (0.63–0.81) | <0.001 |
| Age at treatment (per 5-yr increment) | 1.01 (0.93–1.09) | 0.8 | 1.04 (0.94–1.16) | 0.4 |
| ln(iPSA) | 0.88 (0.77–1.01) | 0.064 | 0.85 (0.7–1.03) | 0.095 |
| Gleason grade group (reference: GG1) b | ||||
| GG 2 | 1.32 (0.62–2.81) | 0.5 | 5.14 (0.64–41.22) | 0.12 |
| GG 3 | 2.03 (0.95–4.30) | 0.066 | 9.16 (1.17–71.85) | 0.035 |
| GG 4 | 1.89 (0.96–3.69) | 0.064 | 7.94 (1.06–59.14) | 0.043 |
| GG 5 | 2.35 (1.19–4.65) | 0.014 | 17.24 (2.33–127.45) | 0.005 |
| Clinical T stage (reference: cT1c) c | ||||
| cT2a | 1.50 (0.95–2.36) | 0.083 | 1.08 (0.56–2.05) | 0.8 |
| cT2b | 1.00 (0.64–1.57) | >0.9 | 0.91 (0.46–1.8) | 0.8 |
| cT2c | 0.91 (0.56–1.48) | 0.7 | 0.77 (0.37–1.61) | 0.5 |
| cT3a | 1.33 (0.88–2.03) | 0.18 | 1.10 (0.58–2.08) | 0.8 |
| cT3b | 1.22 (0.77–1.94) | 0.4 | 1.19 (0.62–2.28) | 0.6 |
| cT4 | 2.27 (1.22–4.23) | 0.010 | 1.87 (0.84–4.13) | 0.12 |
| External beam radiation therapy + BT | ||||
| Interval to biochemical failure a | 0.87 (0.77–0.99) | 0.031 | 0.81 (0.69–0.94) | 0.008 |
| Age at treatment (per 5-yr increment) | 1.00 (0.85–1.18) | >0.9 | 0.96 (0.79–1.17) | 0.7 |
| ln(iPSA) | 0.83 (0.60–1.15) | 0.3 | 0.78 (0.53–1.15) | 0.2 |
| Gleason grade group (reference: GG1) b | ||||
| GG 2 | 0.29 (0.05–1.78) | 0.18 | 0.46 (0.08–2.64) | 0.4 |
| GG 3 | - | -- | - | - |
| GG 4 | 0.28 (0.09–0.91) | 0.035 | 0.17 (0.04–0.73) | 0.017 |
| GG 5 | 0.33 (0.1–1.05) | 0.060 | 0.54 (0.14–2.11) | 0.4 |
| Clinical T stage (reference: cT1c) c | ||||
| cT2a | 0.35 (0.12–1.01) | 0.053 | 0.24 (0.06–1) | 0.050 |
| cT2b | 0.73 (0.33–1.61) | 0.4 | 0.69 (0.28–1.68) | 0.4 |
| cT2c | 0.63 (0.24–1.65) | 0.3 | 0.88 (0.32–2.47) | 0.8 |
| cT3a | 0.86 (0.4–1.82) | 0.7 | 1.37 (0.64–2.96) | 0.4 |
| cT3b | 0.26 (0.07–0.97) | 0.045 | 0.4 (0.1–1.68) | 0.2 |
| cT4 | - | -- | - | - |
PC = prostate cancer; BT = brachytherapy boost; HR = hazard ratio; CI = confidence interval; GG = grade group; ln(iPSA) = natural log of initial prostate-specific antigen.
Per 1-yr increment.
Per grade group increment.
Per cT stage increment.
This study represents the largest series of clinical outcomes for men with radiorecurrent HRPCa receiving definitive therapy in the modern era. A remarkably high number of DMs were diagnosed within 1 yr of BCR. Further highlighting the aggressive natural history, 5-yr estimates of PCSM were nearly 30%. The only consistent predictor of DM and PCSM across both EBRT and EBRT + BT, as well as in the entire cohort, was interval to BCR, with longer interval associated with lower risk of DM and PCSM. This finding is consistent with prior reports for all-risk prostate cancer [1,6].
Early DMs after BCR may be manifestations of occult micrometastatic disease present at the time of RT that ultimately progressed after cessation of ADT (or with castrate resistance). A proportion of these DMs may also simply reflect rapid progression from local recurrence to distant failure. The latter may also explain the separation of the DMFS curves (and rates) beyond the 1-yr mark: it is possible that greater initial local tumor eradication by the BT boost [7] may reduce these “late waves” of DMs [8], which would explain, at least in part, the overall lower DM rate observed after EBRT + BT compared to EBRT. As advanced imaging techniques, such as prostate-specific membrane antigen positron emission tomography/computed tomography, are further integrated into initial staging, identification of occult DM in high-risk populations may enhance upfront patient selection and treatment decision-making [9,10].
There are several limitations to this study. There are likely to be significant selection biases that impacted the choice of treatment with EBRT or EBRT + BT, such as performance status and comorbidities; these factors may have implications for a patient’s life expectancy, ability to tolerate upfront systemic therapies, and ability to undergo salvage therapies. In effect, the rates of DM and PCSM presented—and any differences observed between treatments—are not solely reflective of outcomes after each treatment but may also reflect potential comorbidity confounders. Unfortunately, medical comorbidity data were not available. In addition, owing to the nature of multivariable analyses, the aggregate clinical picture of where each patient fell on the high-risk spectrum was not included in these models or outcomes, so treatment selection bias for very high-risk patients could also have influenced the predictive model results. Furthermore, details regarding diagnostic evaluation at the time of BCR, such as timing and use of imaging studies to detect local or distant recurrence, or biopsies of the prostate and/or potential metastatic sites, were not available. Thus, we cannot know the distribution of biochemical failures that were attributable to local recurrence, DM, or a combination of the two. This also precludes our ability to report the percentage of patients who received local salvage among those who were appropriate candidates. Further study is needed to better understand which men with radiorecurrent HRPCa might benefit from local salvage therapy.
Overall, our findings indicate that recurrence after RT for HRPCa follows an aggressive clinical course, as many these patients develop early metastases and may harbor micrometastatic disease. The markedly high rate of PCSM probably reflects that HRPCa that recurs after both ADT and local definitive treatment is biologically aggressive disease. Further studies are warranted to better evaluate whether advanced imaging and risk stratification tools can change the natural history after recurrence, and potentially even improve clinical outcomes by informing the initial treatment strategy for high-risk patients.
Supplementary Material
Funding/Support and role of the sponsor:
This work was supported by the American Society for Radiation Oncology and the Prostate Cancer Foundation, as well as the Jonsson Comprehensive Cancer Foundation and a charitable donation from the Rosenson Family Trust. The sponsor played a role in analysis of the data.
Footnotes
Financial disclosures: Amar U. Kishan certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Nicholas G. Nickols has received grants from Veterans Affairs, Prostate Cancer Foundation, STOP Cancer Foundation, Janssen, Bayer, Progenics, and Varian; and personal fees from Gene Sciences, Inc. Matthew B. Rettig has received research grants from Novartis, Johnson & Johnson, Merck, Astellas, and Medivation; is a member of speaker bureaus for Johnson & Johnson and Bayer; and is a consultant for Constellation, Ambrx, and Amgen. Daniel E. Spratt is an advisory board member for Blue Earth Diagnostics, Janssen, and AstraZeneca. Jonathan D. Tward has received research funding from Bayer and Myriad; acts in a consulting role for Bayer, Blue Earth, Merck, and Astellas; and participates in a speaker bureau for Blue Earth. Jeffrey J. Tosoian holds a leadership role with equity interest in LynxDx, Inc. Paul C. Boutros is a member of the scientific advisory boards of BioSymetrics Inc. and of Intersect Diagnostics Inc. Amar U. Kishan has acted in an advisory role for Janssen and a consulting role for mPROS; has received grants from the Prostate Cancer Foundation; and has received honoraria from Varian and ViewRay. The remaining authors have nothing to disclose.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eururo.2021.04.035.
Data sharing statement:
Owing to multi-institutional data sharing policies and restrictions, research data are not available for sharing at this time.
References
- [1].Zumsteg ZS, Spratt DE, Romesser PB, et al. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur Urol 2015;67:1009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965–74. [DOI] [PubMed] [Google Scholar]
- [3].Spratt DE, McHugh DJ, Morris MJ, Morgans AK. Management of biochemically recurrent prostate cancer: ensuring the right treatment of the right patient at the right time. Am Soc Clin Oncol Educ Book 2018;38:355–62. [DOI] [PubMed] [Google Scholar]
- [4].Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol 2017;36:446–53. [DOI] [PubMed] [Google Scholar]
- [5].Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–26. [Google Scholar]
- [6].Johnson S, Jackson W, Li D, et al. The interval to biochemical failure is prognostic for metastasis, prostate cancer-specific mortality, and overall mortality after salvage radiation therapy for prostate cancer. Int J Radiat Oncol 2013;86:554–61. [DOI] [PubMed] [Google Scholar]
- [7].Morris WJ, Tyldesley S, Rodda S, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017;98:275–85. [DOI] [PubMed] [Google Scholar]
- [8].Coen JJ, Zietman AL, Thakral H, Shipley WU. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol 2002;20:3199–205. [DOI] [PubMed] [Google Scholar]
- [9].Calais J, Kishan AU, Cao M, et al. Potential impact of 68Ga-PSMA-11 PET/CT on the planning of definitive radiation therapy for prostate cancer. J Nucl Med 2018;59:1714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol 2016;13:226–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Owing to multi-institutional data sharing policies and restrictions, research data are not available for sharing at this time.