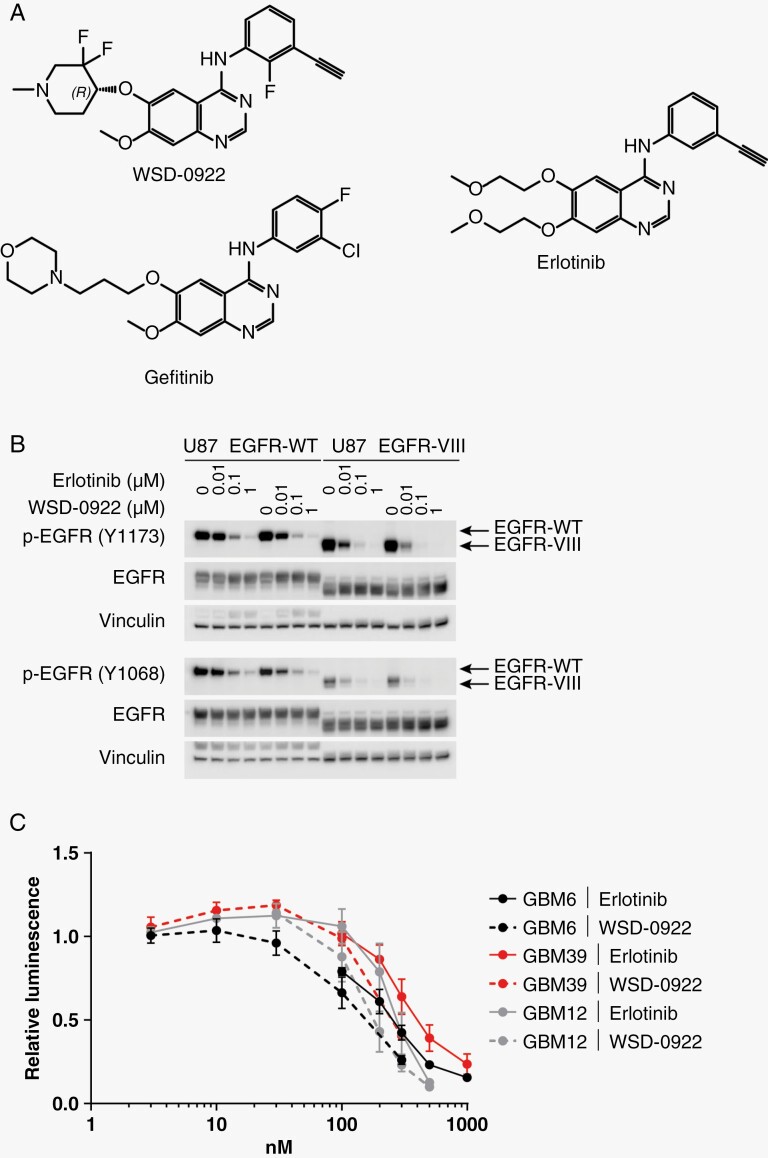
Figure 1.
Biochemical and in vitro characterization of WSD-0922. (A) The chemical structure of WSD-0922 compared to the first-generation epidermal growth factor receptor (EGFR) inhibitors erlotinib and gefitinib. Structures were generated using ChemDraw 21.0.0. (B) Western blot showing the dose-dependent effect of erlotinib and WSD-0922 on pEGFR in U87 cell lines stably overexpressing WT or vIII EGFR. Antibodies against Tyr 1173 and Tyr 1068 pEGFR were used for immunoblotting. (C) Cell TiterGlo was used to assess the impact of erlotinib and WSD-0922 on the viability of GBM patient derived xenograft lines in ex vivo culture. Lines were treated with the indicated doses of drug for 14 days before luminescence readings were measured. Some data points were omitted to allow for curve fitting and determination of IC50 values.