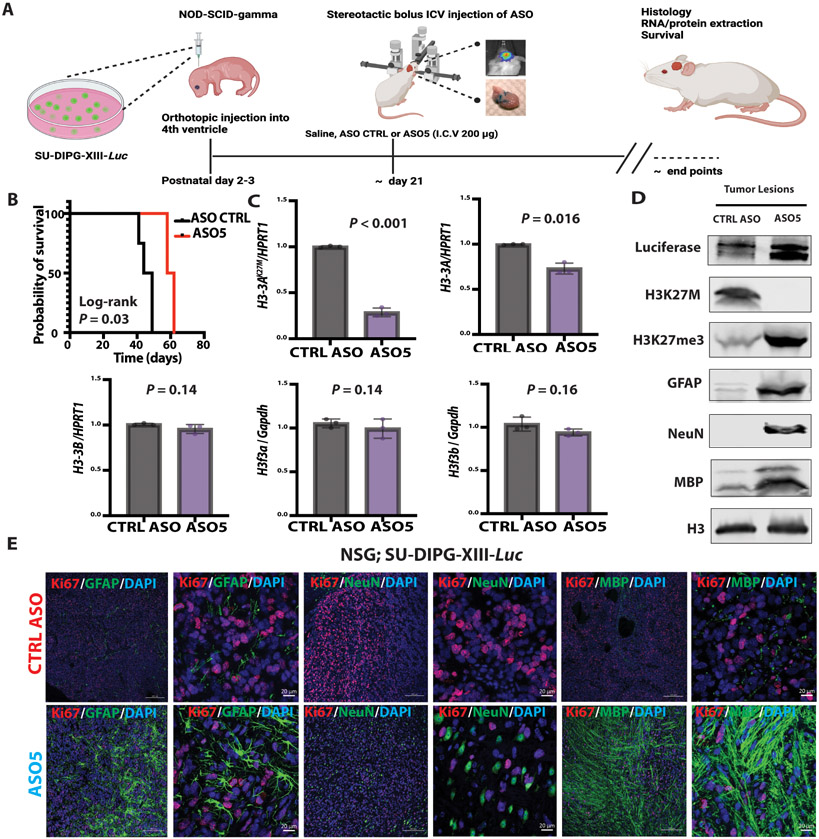
Fig. 6. ICV administration of ASO at the time of tumor onset induced human-specific astrocyte, neuron, and oligodendrocyte differentiation, decreased tumor cell proliferation, and extended the latency of tumor growth in a patient-derived xenograft mouse model.
(A) A single dose (200 μg) of lead ASO or CTRL ASO in saline was stereotactically injected ICV at the time of tumor onset (~day 21) in SU-DIPG-XIII-Luc xenografted mice; (B) Kaplan-Meier survival analysis after CTRL ASO (N = 5) or ASO5 (N = 5) treatment; (C) Summary of data of mRNA expression detected by RT-qPCR (n = 3) of H3-3AK27M allele, total H3-3A, and H3-3B, normalized to HPRT1, and of endogenous murine H3f3a and H3f3b normalized to Gapdh expression (N = 3 mice per group); (D) Immunoblot for histones, transduced luciferase, and differentiation markers (GFAP, NeuN, and MBP) in tissue samples prepared from CTRL ASO-treated or ASO5-treated tumor lesions; (E) Representative IF images showing GFAP+, NeuN+ and MBP+ cells (green) and proliferation by Ki67 staining (red) after CTRL ASO (top row) or ASO5 treatment (bottom row); DAPI staining shows nuclei (blue) (low-magnification images: scale bar, 200 μm; high-magnification images: scale bar, 20 μm). For RT-qPCR experiments, the measurements for each experimental group/treatment were analyzed by Welch’s two-sample t-test, and data are presented as means ± SEM. Probability of survival was compared using log-rank survival estimate.