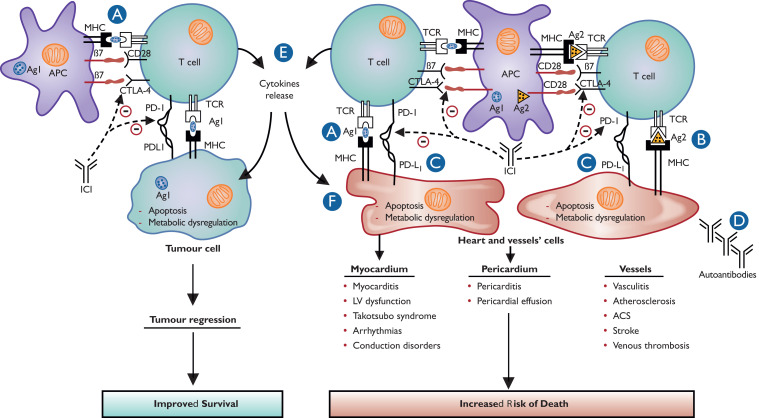
Figure 2.
Proposed pathophysiology and mechanisms of cardiovascular immune-related adverse events. (A) T-cell targeting cardiovascular cells might result from cross-reactivity of tumour and cardiovascular tissues antigens. Tumour and cardiovascular antigens might be similar or with common epitopes. (B) T-cell targeting cardiovascular cells might result from important immune reaction and decrease in cell self-tolerance, which can lead to self-antigen recognition, especially if they had been modified by previous cardiovascular injury. (C) The use of immune checkpoint inhibitor in the context of programmed death ligand 1 overexpression on cardiovascular cells might also contribute to T-cell mediated myocardial, vascular, and pericardial injury related to T-cell infiltration. This overexpression of programmed death ligand 1 is protective in processes such as myocardial ischaemia reperfusion or atherosclerosis. (D) Though autoantibodies targeting cardiac troponin were only detected in pre-clinical models, the hypothesis that activation of the immune system might upregulate pre-existing autoantibodies, especially in predisposed patients, is not completely excluded. (E) The release of pro-inflammatory cytokines secondary to an immune response dysregulated by immune checkpoint inhibitors may cause cytokine release syndrome resulting in myocardial injury. However, cytokine release syndrome is rarely observed with immune checkpoint inhibitor therapy and is more frequent with CAR T-cell therapy. (F) Dysregulation of myocardial metabolism induced by smouldering inflammation is a possible mechanism of myocardial dysfunction after immune checkpoint inhibitor administration.