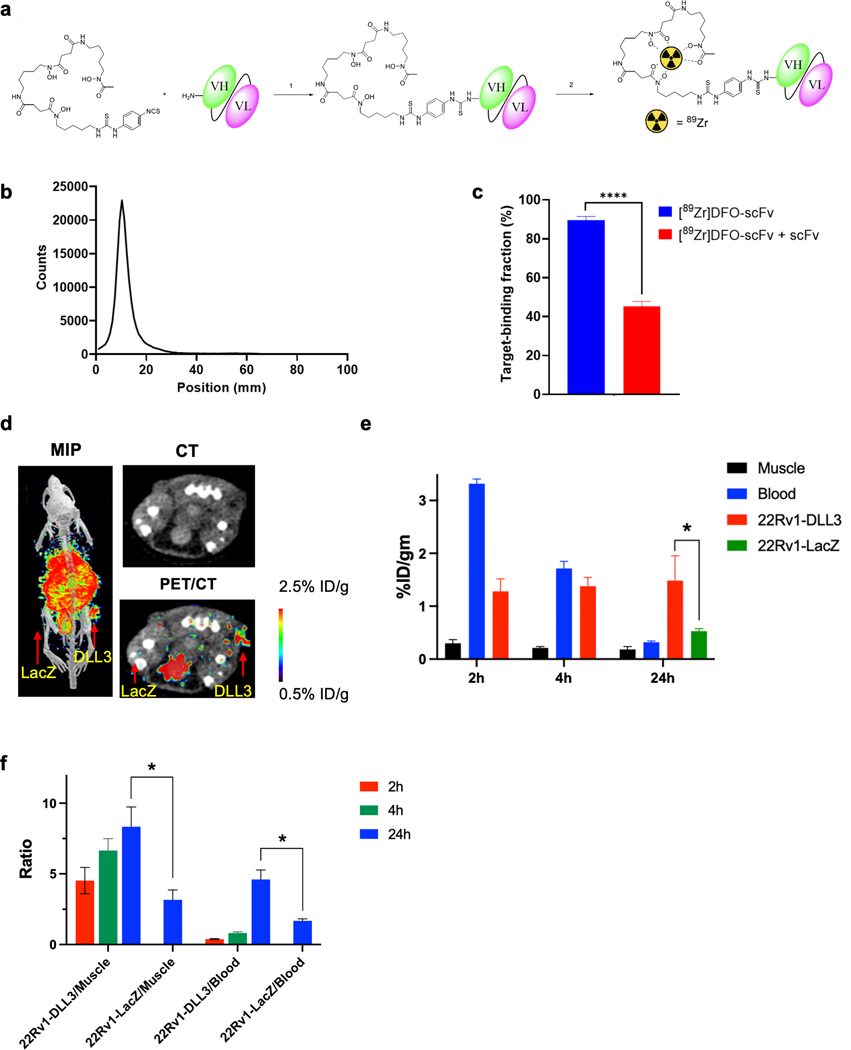
Figure 4. [89Zr]DFO-DLL3-scFv ImmunoPET Imaging of DLL3+ Tumor Xenografts.
a. Schematic for [89Zr]DFO-scFv generation. b. iTLC analysis of purified [89Zr]DFO-scFv. c. Magnetic bead–based radioligand binding assay for DLL3 target binding fraction in the absence or presence of unlabeled DLL3 scFv. **** p < 0.0001. d. Maximum-intensity projection (MIP) μPET/CT, transaxial CT, and transaxial μPET/CT slices obtained 24h after administration of [89Zr]DFO-scFv in 22RV1-LacZ and 22RV1-DLL3 dual tumor xenografts. e. Biodistribution data of [89Zr]DFO-scFv from selected tissues from 2h to 24h in mice bearing 22Rv1-LacZ and 22RV1-DLL3 dual tumors. f. Tumor/blood and tumor/muscle ratio of [89Zr]DFO-scFv from 2h to 24h in 22Rv1-LacZ and 22RV1-DLL3 dual tumors. *p < 0.05.