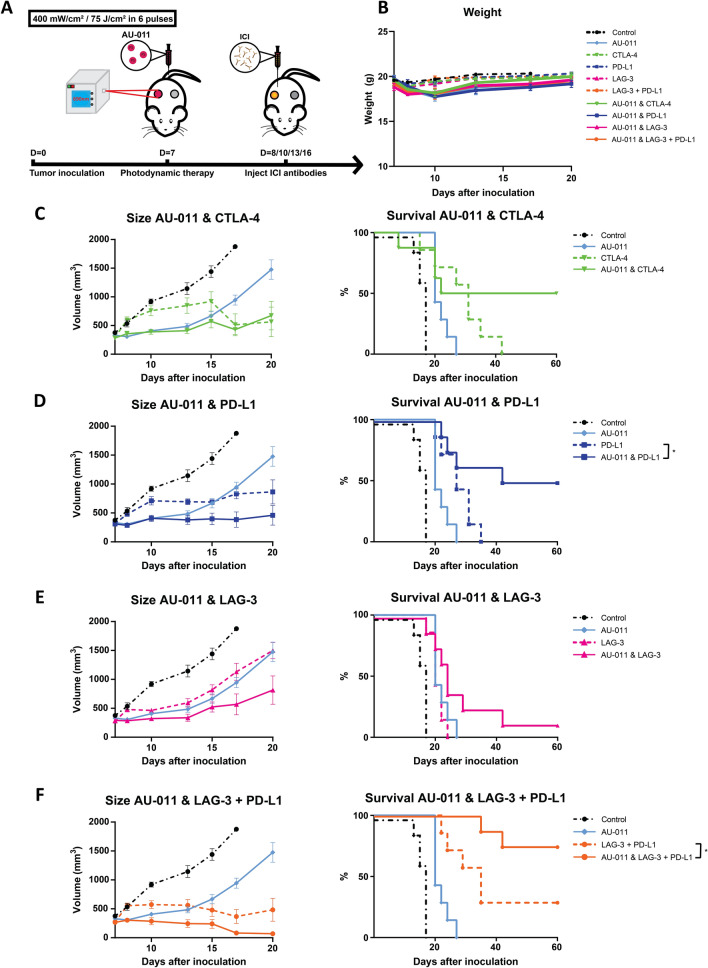
Fig. 6.
AU-011 treatment enhances immune checkpoint inhibition in primary and distant tumors. A C57BL/6 mice were inoculated with 0.5 × 106 MC38 cells in the right flank (primary tumor injection site) and 0.25 × 106 MC38 cells in the left flank (distant tumor injection site). At 7 days post inoculation, when tumors were established (125 mm3), the mice were injected with 100 µg of AU-011 intravenously into the tail vein. The primary tumors on the right flank were then illuminated with NIR light (690 nm) at a DLI of 12 h with 400 mW/cm2 for 75 J/cm2. At days 8, 10, 13 and 16 post inoculation, immune checkpoint inhibitory antibodies CTLA-4 (200 µg per administration), PD-L1 (200 µg per administration), LAG-3 (200 µg per administration) or LAG-3 together with PD-L1 (150 µg of each antibody per administration) were injected intraperitoneally, after which the animals were monitored over time and compared to control (without AU-011, light and immune checkpoint inhibitory antibodies). B Animal weight of animals corresponding to the protocol as described. The total tumor burden (cumulative of the primary and distant tumors) and survival curves of animals treated with C AU-011 and CTLA-4, D AU-011 and PD-L1, E AU-011 and LAG-3 and F AU-011 and LAG-3 with PD-L1, corresponding to the protocol as described. Statistical analysis was performed using the Mantel-Cox test. (*p < 0.05; mean ± SEM; n ≥ 8)