Abstract
Background:
Dysfunction in major stress response systems during the acute aftermath of trauma may contribute to risk for developing PTSD. The present study investigated how PTSD diagnosis and symptom severity, depressive symptoms, and childhood trauma uniquely relate to diurnal neuroendocrine secretion (cortisol and alpha-amylase rhythms) in women who recently experienced interpersonal trauma compared to non-traumatized controls.
Method:
Using a longitudinal design, we examined diurnal cortisol and alpha-amylase rhythms in 98 young women (n=57 exposed to recent interpersonal trauma, n=41 non-traumatized controls). Participants provided saliva samples and completed symptom measures at baseline and 1-, 3-, and 6-month follow-up.
Results:
Multilevel models revealed lower waking cortisol predicted development of PTSD in trauma survivors and distinguished at-risk women from non-traumatized controls. Women with greater childhood trauma exposure exhibited flatter diurnal cortisol slopes. Among trauma-exposed individuals, lower waking cortisol levels were associated with higher concurrent PTSD symptom severity. Regarding alpha-amylase, multilevel models revealed women with greater childhood trauma exposure exhibited higher waking alpha-amylase and slower diurnal alpha-amylase increase.
Conclusions:
Results suggest lower waking cortisol in the acute aftermath of trauma may be implicated in PTSD onset and maintenance. Findings also suggest childhood trauma may predict a different pattern of dysfunction in stress response systems following subsequent trauma exposure than the stress system dynamics associated with PTSD risk; childhood trauma appears to be associated with flattened diurnal cortisol and alpha-amylase slopes, as well as higher waking alpha-amylase.
Keywords: PTSD, interpersonal trauma, cortisol, alpha-amylase
Introduction
Posttraumatic stress disorder (PTSD) is a common, debilitating disorder (Kilpatrick et al., 2013) linked to significant functional impairment and economic burden (Bothe et al., 2020). Women exposed to interpersonal violence (IPV) are at an especially high risk of developing PTSD (Shalev et al., 2019). Identifying biomarkers associated with PTSD in this population may aid in reducing the public health burden through early identification and intervention for those at greatest risk of developing PTSD.
Dysfunction in stress response systems, including the hypothalamic-pituitary-adrenal (HPA) axis, sympathetic nervous system (SNS), and parasympathetic nervous system has been linked to PTSD (Agorastos et al., 2020; Morris et al., 2012; Pole, 2007), and may represent a biomarker for PTSD risk. The present study focuses on HPA and SNS dysfunction. Prior research has found lower daily cortisol output in individuals with PTSD compared to controls (Meewisse et al., 2007; Miller et al., 2007; Morris et al., 2012; Speer et al., 2019; Yehuda, 2002). Moreover, greater time since posttraumatic stress symptom onset is associated with lower daily cortisol output (Morris et al., 2012). Theoretical models propose trauma exposures progressively attenuate cortisol secretion, which increases risk for developing PTSD following subsequent traumas (Steudte-Schmiedgen et al., 2016) via hypothesized effects on trauma memory consolidation (de Quervain et al., 2009), context processing (Liberzon & Abelson, 2016), and circadian rhythm (Agorastos et al., 2020). Consistent with theory, lower circulating cortisol levels are associated with increased risk for developing PTSD (Morris et al., 2016; Morris & Rao, 2013), and glucocorticoid administration in recent trauma survivors reduces risk for PTSD onset (Sijbrandij et al., 2015). However, whether and in what manner blunted cortisol contributes to PTSD onset in recent IPV survivors remains unclear.
In contrast to decreased HPA activity, research suggests individuals with PTSD exhibit increased SNS activity relative to non-traumatized controls (NTCs) and trauma-exposed individuals without PTSD (Buckley & Kaloupek, 2001; O’Donnell et al., 2004; Pole, 2007). Heightened SNS activity has been implicated in trauma memory consolidation models of PTSD (de Quervain et al., 2009), though this role has not always been supported by prospective (Morris & Rao, 2013) or pharmacological intervention studies (Sijbrandij et al., 2015). Nevertheless, meta-analytic evidence indicates elevated heart rate within 72 hours of trauma exposure corresponds to higher PTSD risk (Morris et al., 2016). Emerging evidence suggests individuals with PTSD exhibit higher alpha-amylase levels, an indicator of SNS activity (Nater & Rohleder, 2009), than non-traumatized controls (Nicholson et al., 2014). Moreover, greater intrusive and hyperarousal symptoms are associated with higher morning alpha-amylase levels in adolescent trauma survivors (Keeshin et al., 2015). Together, findings suggest alpha-amylase is associated with PTSD, but it remains unclear whether alpha-amylase represents a biomarker for subsequent PTSD onset and/or maintenance.
Few studies have evaluated diurnal variation in both HPA and SNS secretion in relation to PTSD. Cross-sectional research suggests individuals with PTSD exhibit flatter diurnal cortisol slope compared to controls, whose cortisol levels rise after waking and drop throughout the day (Nicolson & Ponnamperuma, 2019; Thompson et al., 2015). Meta-analyses have demonstrated a pattern of lower waking (Miller et al., 2007) or daily (Morris et al., 2012) cortisol levels and higher afternoon/evening levels in individuals exposed to trauma compared to controls. This is consistent with research demonstrating flatter diurnal cortisol slope corresponds to negative physical and mental health outcomes (see Adam et al., 2017 for a review). One study, which examined diurnal variation in alpha-amylase, found individuals with PTSD exhibited a sharp increase in alpha-amylase levels after waking (compared to a decrease after waking in controls), with positive associations between alpha-amylase levels and PTSD symptoms (Thoma et al., 2012). Adam and colleagues (2017) theorize that psychosocial stressors can both trigger and maintain dysregulation in circadian processes (Hall et al., 2004; Sadeh et al., 2004; Van Reeth et al., 2000), which can have cascading effects on many regulatory systems (e.g., biological, behavioral). These changes can, in turn, negatively affect physical and mental health (Adam et al., 2017). However, the majority of this research has been cross-sectional and has not accounted for other important determinants of dysfunction in stress response symptoms following trauma exposure.
Several risk factors may influence diurnal HPA and SNS secretion in PTSD. Trauma history has been linked to both altered HPA functioning and risk of PTSD onset (Delahanty & Nugent, 2006). Moreover, meta-analytic findings highlight the need to examine the effect of co-occurring depressive symptoms given findings that depression and PTSD exert opposing effects on cortisol levels (Morris et al., 2012). Research also suggests age may moderate the direction of the relationship between stress response system dysfunction and PTSD (Morris et al., 2016). Few studies have examined these factors simultaneously. Elucidating the relations between these variables and diurnal cortisol/alpha-amylase secretion could assist in early identification and intervention for those at greatest risk of developing PTSD or other posttraumatic psychopathology.
Using an innovative, longitudinal design, the present study examined the dynamics of diurnal cortisol and alpha-amylase rhythms in women following recent IPV compared to healthy NTCs. The primary aim was to understand the independent relations between diurnal cortisol and alpha-amylase rhythms and PTSD, depressive symptoms, and childhood. We hypothesized that flatter diurnal cortisol slope and steeper alpha-amylase slope would correspond to increased risk of concurrent and subsequent PTSD onset and symptom severity.
Method
Participants
The present sample comprised 98 women aged 18–30 (inclusive). Of these, 57 reported exposure to IPV, defined as any incident involving physical and/or sexual assault, within approximately three months of their baseline assessment (range of days since index trauma at baseline: 5 −101, M=45.32, SD=24.74). A comparison group of 41 non-traumatized controls (NTC) included healthy women with no IPV exposure. For the IPV group, exclusion criteria were: current major depressive disorder (MDD) that preceded their index trauma; PTSD resulting from an index trauma that occurred prior to the three-month window; current substance use disorder; or history of bipolar or psychotic disorder. All participants were administered the Structured Clinical Interview (SCID) for DSM-IV (First et al., 2005) to assess for mood and substance use disorders at baseline. PTSD was evaluated in the IPV group at each assessment via the Clinician Administered PTSD Scale (CAPS) for DSM-IV (Blake et al., 1995). For NTCs, exclusion criteria were: lifetime IPV exposure, any trauma exposure in the previous year, and history of mood or anxiety disorder or PTSD. Additional exclusion criteria for all participants were: serious health conditions which influence HPA or SNS activity, pregnancy, or current use of prescription or non-prescription drugs which affect HPA or SNS activity.
Protocol
Details of the study protocol have been described previously (Morris et al., 2020). Briefly, participants were recruited through online advertisements, and the IPV group was also recruited from a local hospital and local agencies coordinating services for domestic violence and sexual assault survivors. Participants completed assessments and provided saliva samples at baseline and at 1– 3-, and 6-month follow-up. At each assessment, the CAPS interview (Blake et al., 1995) and the SCID mood modules (First et al., 2005) were administered. Participants also completed questionnaires via the secure web-based Research Electronic Data Capture (REDCap) platform (Harris et al., 2009) and provided saliva samples over two consecutive days at each time point.
Measures
Prior trauma exposure
The Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 1994), a 28-item self-report measure, assessed exposure to childhood abuse and neglect in all participants. Coefficient alpha for the CTQ in this sample was 0.87.
Depressive symptoms
The Beck Depression Inventory, second edition (BDI-II) (Beck et al., 1996), a 21-item self-report measure, assessed depressive symptoms in all participants. Coefficient alphas in this sample were excellent (range: 0.92 to 0.94).
Posttraumatic Stress Symptoms and Disorder
The clinician-administered CAPS interview for DSM-IV (Blake et al., 1995) assessed overall posttraumatic stress symptom severity, symptom cluster severity (i.e., re-experiencing, avoidance, hyperarousal), and PTSD diagnostic status (using the ‘F1/I2’ rule) at each assessment (Weathers et al., 1999) in the IPV group. Inter-rater reliability in this sample was excellent for CAPS total (ICC = .997) and CAPS subscale (ICC’s > .993) scores.
Cortisol and Alpha-Amylase
Cortisol and alpha-amylase levels were determined by saliva samples collected using acid-free cotton swabs (Sarstedt Inc., Newton, NC). Participants were instructed to provide 5 saliva samples (upon awakening, +30 minutes after awakening, before lunch, ~3 pm, ~9 pm) per day over two consecutive weekdays. Participants were instructed to refrain from brushing their teeth, eating, drinking caffeine, or engaging in rigorous exercise within 30 minutes of each sample, and not to drink alcohol or use tobacco products during collection days. Participants recorded the timing of each saliva sample and stored samples in their home freezer before returning them at their next visit. Cortisol levels were determined by commercial chemiluminescence immunoassay (Kirschbaum & Hellhammer, 1989). Alpha-amylase levels were determined by a quantitative enzyme kinetic method (Nater et al., 2007). Intra- and inter-assay coefficients of variation for these assays were below 6%. Participants also completed paper-and-pencil measures, including an item which asked participants to report the number of hours they slept the night before each saliva sample collection day.
Data Analysis
Distributional properties were examined for all variables, and cases were screened for outliers. The findings reported below were unchanged after winsorizing cortisol and alpha-amylase outliers (≥ 3 SD). Salivary cortisol and alpha-amylase levels were natural log-transformed due to their right-skewed distributions. The Statistical Package for the Social Sciences (SPSS; Chicago, IL version 27) was used for data management; descriptive statistics; and the calculation of total-R2, a measure of the total proportion reduction in the total outcome variance across all levels of analysis. Missing cortisol and alpha-amylase data were handled using maximum-likelihood estimation. Multilevel models (MLMs) were specified using hierarchical linear models (HLM v. 8) to examine how within-individual changes in diurnal cortisol or alpha-amylase levels varied across time of day (level 1), across assessments (level 2), and between individuals (level 3) (Raudenbush et al., 2019). MLMs in the full sample separately examined the influence of age (level 3), childhood trauma (level 3), mean depressive symptoms (level 3), days since baseline (level 2), assessment-level depressive symptoms (level 2), PTSD diagnostic status at each assessment (level 2), and minutes from waking (level 1) on diurnal cortisol or alpha-amylase levels. Of note, the continuous minutes from waking variable, rather than an ordinal variable indexing each sample (i.e., 1–5), was entered as a level-1 predictor in order to examine the diurnal cortisol and alpha-amylase slopes because time since waking strongly affects neuroendocrine markers (Nicolson & Ponnamperuma, 2019; Thompson et al., 2015). Predictors from these separate MLMs that were significantly associated with changes in diurnal cortisol or alpha-amylase were then entered into a combined model to examine their independent effects. The combined model including diagnostic status, depressive symptoms, age, and childhood trauma as predictors of diurnal cortisol is presented below.
Level 1 model (within day):
Level 2 model (between assessments):
Level 3 model (between person):
In this equation, Cortisol denotes natural log-transformed cortisol levels for each participant on a specific collection day at a specific assessment time point. Predictors of waking cortisol (intercept) and linear change in cortisol across the day (slope) included: Age; Diagnostic Status (determined at each assessment: either PTSD, IPV without PTSD, or NTC); assessment-level Depression (to assess the within-individual effect of depressive symptoms); mean Depression (averaged across assessments to assess the between-person effect of depressive symptoms); and Childhood Trauma (CTQ total score). Of primary interest in the full sample were the cross-level interactions between Diagnostic Status and Minutes (β11), assessment-level Depression and Minutes (β12), mean Depression and Minutes (ϒ102), and Childhood Trauma and Minutes (ϒ101). Diagnostic status could change from one assessment to the next within the IPV group.
Next, MLMs within the IPV group simultaneously examined the influence of PTSD symptom severity (level 2), assessment-level depressive symptoms (level 2), and mean depressive symptoms (level 3) on diurnal cortisol or alpha-amylase levels. Of primary interest within the IPV group were the depressive symptoms (BDI-II) X Minutes from waking and PTSD symptom severity (CAPS) X Minutes from waking interactions.
Identical MLMs in the full sample and within the IPV group were specified to evaluate predictors of waking alpha-amylase and linear change in alpha-amylase across the day.
Finally, the analyses were repeated using time-lagged diagnostic and symptom measures to investigate whether cortisol and alpha-amylase predicted PTSD diagnostic status and/or depressive symptom severity at the following assessment using the approach described by Shirtcliff and Essex (2008).
For all MLMs, simple slopes analysis was used to probe significant interactions (Preacher et al., 2006), and interaction patterns are presented for higher (+1 SD) and lower (−1 SD) values of continuous independent variables. To account for multiple hypothesis tests, we used the Benjamini-Hochberg false discovery rate correction to control for Type I errors by adjusting the p-value based on the number of significant results in a family of tests (Benjamini & Hochberg, 1995).
Results
Participant Characteristics
Demographic and clinical characteristics are presented in Table 1 separately for women within the IPV group who met PTSD criteria at any point during the study (IPV + PTSD), women within the IPV group who never met criteria for PTSD during the study (IPV without PTSD), and NTC women. The IPV + PTSD group reported greater depressive symptoms and childhood trauma exposure compared to the IPV without PTSD group. The IPV without PTSD group reported greater depressive symptoms and childhood trauma exposure than the NTC group. Racial/ethnic differences between groups revealed a higher proportion of non-Hispanic White/Caucasian participants in the NTC compared to the IPV groups. Additionally, the NTC group reported greater educational attainment than either IPV group. The groups did not differ in age or marital status.
Table 1.
Descriptive and clinical characteristics for recent interpersonal violence survivors who met diagnostic criteria for Posttraumatic Stress Disorder at any point during the study (IPV + PTSD), those who did not (IPV without PTSD), and the Non-Traumatized Control (NTC) group
| Mean (SD) or n (%) | Group Comparison | |||
|---|---|---|---|---|
|
| ||||
| IPV + PTSD (n = 21) | IPV without PTSD (n = 36) | NTC (n = 41) | F or χ 2 | |
|
|
||||
| Sociodemographic | ||||
| Age (years) | 24.14 (2.61) | 23.64 (3.67) | 24.10 (3.26) | 0.24 |
| Race/Ethnicity | 11.09* | |||
| White/Caucasian | 10 (47.62) | 22 (61.11) | 33 (80.49) | |
| Black/African-American | 10 (47.62) | 9 (25.00) | 5 (12.20) | |
| Asian | 1 (4.76) | 5 (13.89) | 3 (7.32) | |
| Hispanic | 0 (0.00) | 4 (11.11) | 0 (0.00) | 7.35* |
| Education (years) | 14.76 (1.92)a | 14.77 (1.96)a | 16.68 (2.26)b | 9.80*** |
| Marital Status | 10.69 | |||
| Single | 17 (80.95) | 29 (80.56) | 29 (70.73) | |
| Married | 1 (4.76) | 0 (0.00) | 6 (14.63) | |
| Engaged | 1 (4.76) | 1 (2.78) | 0 (0.00) | |
| Living with partner | 1 (4.76) | 3 (8.33) | 1 (2.44) | |
| Divorced | 0 (0.00) | 0 (0.00) | 1 (2.44) | |
| Depressive symptoms (BDI-II) | 22.05 (10.49)a | 11.67 (9.56)b | 4.95 (5.75)c | 26.71*** |
| Childhood trauma (CTQ) | 57.25 (20.95)a | 43.12 (14.66)b | 30.92 (6.37)c | 23.95*** |
Note. IPV = interpersonal violence; PTSD = posttraumatic stress disorder; NTC = non-traumatized control.
p<.001
p<.01
p<.05. Within rows, values with different superscripts differ significantly at p<.05
Preliminary Analyses of Diurnal Cortisol and Alpha-Amylase Secretion
Salivary cortisol levels (averaged across collection days) are presented separately for each group in Figure 1 at baseline (Panel A) and 1-month follow-up (Panel B). MLMs revealed that cortisol levels declined significantly throughout the day (b=−.0023, SE=.0001, p<.001) across all participants, controlling for days since baseline. Age moderated changes in cortisol levels throughout the day (b=−0.0001, SE=0.00002, p=.002); older participants demonstrated a steeper diurnal decline in cortisol than younger participants. As time (days from baseline) elapsed, waking cortisol levels declined (b=−0.0041, SE=0.0009, p<.001), and diurnal cortisol slopes flattened (b=0.000004, SE=0.000002, p=.045). All subsequent MLMs examining diurnal change in cortisol levels included age and days since baseline.
Figure 1. Salivary Cortisol Levels Over Time.
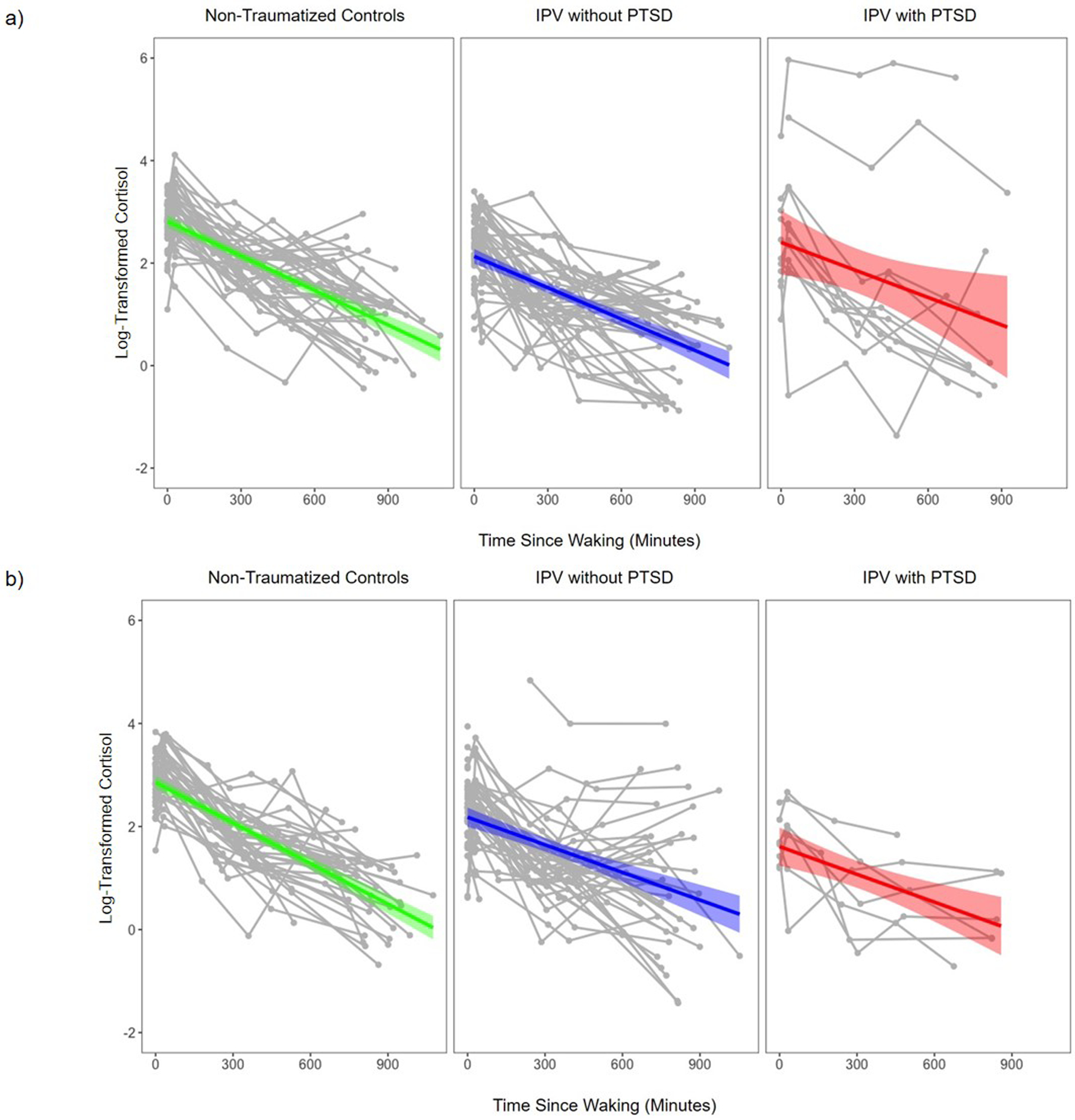
Note. Spaghetti plots depict Assessment Time Point 1 (Panel A) and Assessment Time Point 2 (Panel B) within-person changes in mean salivary cortisol levels for each participant, averaged across both collection days (depicted in gray). The diurnal cortisol trajectory defined by the mean parameters for each group are depicted in green (non-traumatized controls), blue (IPV without PTSD), and red (IPV with PTSD). Shaded area surrounding the trajectory defined by the mean parameters represents the standard error. NTC = non-traumatized control; IPV = interpersonal violence; PTSD = posttraumatic stress disorder.
MLMs revealed that alpha-amylase levels increased throughout the day (b=0.0011, SE=0.0001, p<.001) across all participants, controlling for days since baseline assessment. Age moderated changes in alpha-amylase levels throughout the day (b=0.00004, SE=0.00002, p=.019); older participants demonstrated a steeper diurnal increase in alpha-amylase than younger participants. No other main or interactive effects were significant (p’s≥.171). All subsequent MLMs examining diurnal changes in alpha-amylase levels included age and days since baseline as moderators.
Predictors of Diurnal Cortisol Secretion: Childhood Trauma, Depressive Symptoms, PTSD Diagnostic Status
Concurrent Prediction
Results of MLMs examining predictors of diurnal cortisol secretion in the full sample are presented in the supplementary material. Childhood trauma exposure was significantly associated with both waking cortisol levels and diurnal cortisol slopes. As depicted in Figure 2, simple slope analysis revealed that women with greater childhood trauma exposure exhibited lower waking cortisol levels and flatter diurnal cortisol slopes (b=−0.0019, SE=0.0001, p<.001) compared to women with lower childhood trauma exposure (b=−0.0025, SE0.0001, p<.001). The effect of childhood trauma on waking cortisol levels (b=−0.0137, SE=0.0042, p=.002) and diurnal cortisol slope (b=0.0002, SE=0.000004, p<.001) remained significant when controlling for sample collection day, days from baseline, and age. In contrast, within-person (i.e., assessment-level) depressive symptoms were not associated with waking cortisol levels (b=−0.0001, SE=0.0074, p=.989) or diurnal cortisol slopes (b=−0.00001, SE=0.00002, p=.716). However, between-person mean depressive symptoms were associated with waking cortisol (b=−0.0162, SE=0.0068, p=.019) but not diurnal cortisol slope (b=0.00001, SE=0.00001, p=.357). PTSD diagnostic status was associated with waking cortisol levels and diurnal cortisol slopes. Simple slope analysis revealed lower waking cortisol levels and flatter diurnal cortisol slopes for women with current PTSD (b=−0.0017, SE=0.0002, p<.001) compared to IPV survivors without PTSD (b=−0.0021, SE=0.0001, p<.001) and NTCs (b=−0.0025, SE=0.0001, p<.001). The effect of diagnostic status on waking cortisol level (b=−0.3394, SE=0.0857, p<.001) and diurnal cortisol slope (b=0.0004, SE=0.0001, p<.001) remained significant when controlling for sample collection day, days from baseline, and age.
Figure 2. Effect of Childhood Trauma on Diurnal Cortisol Slope.

Note. CTQ = Childhood Trauma Questionnaire. For illustrative purposes, graph depicts the simple diurnal cortisol slopes (collapsed across all groups) for individuals with lower (−1 SD) and higher (+1 SD) levels of childhood trauma, based on the Childhood Trauma Questionnaire. Additionally, values were defined at 0 and 960 minutes from waking to illustrate the effect of childhood trauma on the diurnal cortisol slope.
Summary of Concurrent Cortisol Findings.
In the combined model (Total-R2=.321), childhood trauma was no longer a significant predictor of waking cortisol levels (b=−0.0076, SE=0.0054, p=.162), but the effect on diurnal cortisol slopes remained significant (b=0.00002, SE=0.00001, p=.001). Within-person depressive symptom levels again did not predict waking cortisol (b=0.0049, SE=0.0072, p=.496) or diurnal cortisol slope (b=−0.00001, SE=0.00002, p=.445). Similarly, between-person mean depressive symptoms did not predict waking cortisol (b=−0.0017, SE=0.0086, p=.844), and the relationship between mean depressive symptoms and diurnal cortisol slope was no longer significant (b=−0.00002, SE=0.00001, p=.052). Whereas PTSD diagnostic status continued to predict waking cortisol levels (b=−0.2164, SE=0.1015, p=.034), it was no longer significantly associated with diurnal cortisol slopes (b=0.0001, SE=0.0001, p=.304) when simultaneously examining the effect of childhood trauma and depressive symptoms. To summarize, when entering diagnostic status, depressive symptoms, and childhood trauma in the same model, a diagnosis of PTSD was associated with lower concurrent waking cortisol, and higher childhood trauma history was associated with a flatter concurrent diurnal cortisol slope1. There were no other significant findings.
Longitudinal Prediction
Results of MLMs examining the relationship between diurnal cortisol secretion and subsequent diagnostic status and depressive symptom severity in the full sample are presented in Table 2. PTSD diagnostic status at the subsequent assessment was significantly associated with waking cortisol levels and diurnal cortisol slopes. Simple slope analysis revealed lower waking cortisol levels and flatter diurnal cortisol slopes in women with subsequent PTSD (b=−0.0018, SE=0.0002, p<.001) compared to IPV survivors without PTSD (b=−0.0021, SE=0.0001, p<.001) and NTCs (b=−0.0025, SE=0.0001, p<.001). The relationship between waking cortisol level (b=−0.5038, SE=0.0942, p<.001) and diurnal cortisol slope (b=0.0003, SE=0.0001, p=.005) and PTSD diagnostic status at the subsequent assessment remained significant when controlling for sample collection day, days from baseline, and age. There was no relationship between waking cortisol levels (b=−0.0087, SE=0.0085, p=.308) or diurnal cortisol slopes (b=−0.0002, SE=0.00002, p=.408) and depressive symptoms at the subsequent assessment (i.e., within-person depressive symptoms).
Table 2.
Longitudinal Diurnal Cortisol Multilevel Models
| Predictors | BDI Model b (SE) | Diagnostic Status Model b (SE) | Combined Model b (SE) |
|---|---|---|---|
|
| |||
| Level 1 (within day) | |||
| Intercept | 2.4575 (0.0764)* | 2.7586 (0.0908)* | 2.8412 (0.1130)* |
| Day | 0.0213 (0.0279) | 0.0196 (0.0264) | 0.0189 (0.0288) |
| Minutes | −0.0023 (0.0001)* | −0.0025 (0.0001)* | −0.0024 (0.0002)* |
| Level 2 (between assessments) | |||
| Days from Baseline | −0.0041 (0.0013)* | −0.0044 (0.0012)* | −0.0043 (0.0012)* |
| Days from Baseline × Minutes | 0.000004 (0.000003) | 0.000003 (0.000002) | 0.000003 (0.000003) |
| BDI at Subsequent Assessment | 0.0087 (0.0085) | 0.0025 (0.0086) | |
| BDI at Subsequent Assessment × Minutes | −0.00002 (0.00002) | −0.00001 (0.00002) | |
| Diagnostic Status at Subsequent Assessment | −0.5038 (0.0942)* | −0.5737 (0.1303)* | |
| Diagnostic Status at Subsequent Assessment × Minutes | 0.0003 (0.0001)* | 0.0001 (0.0002) | |
| Level 3 (between person) | |||
| Age | −0.0078 (0.0212) | 0.0023 (0.0186) | 0.0011 (0.0197) |
| Age × Minutes | −0.00005 (0.00002)* | −0.00004 (0.00002) | −0.0001 (0.00002)* |
| Mean BDI | −0.0181 (0.0068)* | 0.0065 (0.0086) | |
| Mean BDI × Minutes | 0.00001 (0.00001) | −0.00001 (0.00001) | |
| CTQ | −0.0022 (0.0051) | ||
| CTQ × Minutes | 0.00001 (0.00001)* | ||
Note. Day = sample collection day (i.e., day 1 or day 2); Minutes = minutes from waking; BDI = Beck Depression Inventory; CTQ = Childhood Trauma Questionnaire; Diagnostic Status (i.e., current Posttraumatic Stress Disorder [PTSD], interpersonal violence exposure without current PTSD, non-traumatized control) was determined at the subsequent assessment.
p < .05.
Summary of Longitudinal Cortisol Findings.
In the longitudinal model which controlled for childhood trauma and within- and between-person depressive symptom levels (Total-R2=.384), the relationship between waking cortisol level and diagnostic status at the subsequent assessment remained significant (b=−0.5737, SE=0.1303, p<.001), but the relationship between diurnal cortisol slope and diagnostic status at the subsequent assessment was no longer significant (b=0.0002, SE=0.0002, p=.222). In summary, when controlling for childhood trauma and depressive symptoms, lower waking cortisol level was associated with PTSD diagnosis at the subsequent assessment, but diurnal cortisol slope was not2.
Predictors of Diurnal Cortisol Secretion within the IPV Group
Concurrent Prediction
A MLM examined the independent effects of PTSD and depressive symptom severity on diurnal cortisol secretion, controlling for age, days from index trauma, and collection day. Assessment-level PTSD symptom severity was not associated with waking cortisol levels (b=0.0047, SE=0.0044, p=.287) or diurnal cortisol slope (b=−0.00001, SE=0.00001, p=.529). In contrast, mean PTSD symptom severity was negatively associated with waking cortisol level (b=−0.0167, SE=0.0064, p=.012) but not diurnal cortisol slope (b=0.00001, SE=0.00001, p=.109). Assessment-level depressive symptom severity was not associated with waking cortisol (b=−0.0009, SE=0.0089, p=.915) or diurnal cortisol slope (b=0.00002, SE=0.00002, p=.920). Similarly, mean depressive symptom severity was not associated with waking cortisol (b=0.0211, SE=0.0120, p=.086) or diurnal cortisol slope (b=−0.00002, SE=0.00001, p=.206).
Longitudinal Prediction
A MLM examined the relationship between diurnal cortisol secretion and PTSD and depressive symptom severity at the subsequent assessment, controlling for age, days from index trauma, mean PTSD symptom severity, mean depressive symptom severity, and collection day within the IPV group. There was no significant relationship between waking cortisol levels (b=−0.0057, SE=0.0061, p=.352) or diurnal cortisol slopes (b=−0.000001, SE=0.00001, p=.967) and subsequent PTSD symptom severity. Similarly, there was no relationship between waking cortisol levels (b=0.0155, SE=0.0107, p=.153) or diurnal cortisol slopes (b=−0.0001, SE=0.00002, p=.748) and subsequent depressive symptoms.
Summary of Cortisol Findings in the IPV Group.
To summarize, within the IPV group, greater PTSD symptoms were associated with lower concurrent waking cortisol levels3 (Total-R2=.247). There was no significant relationship between waking cortisol or diurnal cortisol slope and PTSD or depressive symptoms at the subsequent assessment.
Predictors of Alpha-Amylase Secretion: Childhood Trauma, Depressive Symptoms, PTSD Diagnostic Status
Concurrent Prediction
Results of MLMs examining predictors of diurnal alpha-amylase secretion in the full sample are presented in Table 3. Childhood trauma exposure was significantly associated with both waking alpha-amylase levels and diurnal alpha-amylase slopes. Simple slope analysis revealed women with greater childhood trauma exposure exhibited higher waking alpha-amylase levels and slower diurnal alpha-amylase increase (b=0.0008, SE=0.0001, p<.001) compared to women with lower childhood trauma exposure (b=0.0012, SE=0.0001, p<.001). The effect of childhood trauma on waking alpha-amylase (b=0.0093, SE=0.0043, p=.033) and diurnal alpha-amylase slope (b=−0.00001, SE=0.000003, p=.001) remained significant when controlling for sample collection day, days from baseline, and age. Within-person (assessment-level) depressive symptoms were not associated with waking alpha-amylase (b=0.0016, SE=0.0068, p=.810) or diurnal alpha-amylase slopes (b=−0.000004, SE=0.00001, p=.754). Between-person mean depressive symptoms were not associated with waking alpha-amylase (b=0.0067, SE=0.0069, p=.332) or diurnal alpha-amylase slopes (b=−0.00001, SE=0.00001, p=.059). PTSD diagnostic status was not significantly associated with waking alpha-amylase levels (b=0.1372, SE=0.0877, p=.119) or diurnal alpha-amylase slopes (b=−0.0001, SE=0.0001, p=.165).
Table 3.
Multilevel Models Predicting Concurrent Diurnal Alpha-Amylase Levels in the Full Sample
| Predictors | BDI Model b (SE) | CTQ Model b (SE) | Diagnostic Status Model b (SE) |
|---|---|---|---|
|
| |||
| Level 1 (within person) | |||
| Intercept | 3.2207 (0.0798)* | 3.2478 (0.0810)* | 3.1158 (0.0979)* |
| Day | 0.0099 (0.0259) | 0.0011 (0.0257) | 0.0104 (0.0249) |
| Minutes | 0.0010 (0.0001)* | 0.0010 (0.0001) | 0.0011 (0.0001)* |
| Level 2 (between assessments) | |||
| Days from Baseline | −0.0013 (0.0009) | −0.0015 (0.0009) | −0.0010 (0.0009) |
| Days from Baseline × Minutes | −0.000001 (0.000002) | −0.000002 (0.000002) | −0.000001 (0.000002) |
| BDI | 0.0016 (0.0068) | ||
| BDI × Minutes | −0.000004 (0.0001) | ||
| Diagnostic Status | 0.1372 (0.0877) | ||
| Diagnostic Status × Minutes | −0.0001 (0.0001) | ||
| Level 3 (between person) | |||
| Age | 0.0334 (0.0221) | 0.0270 (0.0219) | 0.0253 (0.0216) |
| Age × Minutes | 0.00003 (0.00002) | 0.00003 (0.00002)* | 0.00004 (0.00002)* |
| Mean BDI | 0.0067 (0.0069) | ||
| Mean BDI × Minutes | −0.00001 (0.00001) | ||
| CTQ | 0.0093 (0.0043)* | ||
| CTQ × Minutes | −0.00001 (0.000003)* | ||
Note. Day = sample collection day (i.e., day 1 or day 2); Minutes = minutes from waking; BDI = Beck Depression Inventory; CTQ = Childhood Trauma Questionnaire; Diagnostic Status (i.e., current Posttraumatic Stress Disorder [PTSD], interpersonal violence exposure without current PTSD, non-traumatized control) was determined at each assessment.
p < .05.
Summary of Concurrent Alpha-Amylase Findings.
To summarize, greater childhood trauma exposure was associated with higher waking alpha-amylase and slower diurnal alpha-amylase increase compared to lower childhood trauma exposure4 (Total-R2=.103). Depressive symptoms and PTSD diagnosis were not related to waking alpha-amylase or diurnal alpha-amylase slopes.
Longitudinal Prediction
There was no relationship between waking alpha-amylase levels (b=0.1619, SE=0.1113, p=.148) or diurnal alpha-amylase slopes (b=−0.0001, SE=0.0001, p=.316) and PTSD diagnostic status at the subsequent assessment. Similarly, there was no relationship between waking alpha-amylase levels (b=0.0038, SE=0.0089, p=.671) or diurnal alpha-amylase slopes (b<0.000001, SE=0.00002, p=.995) and depressive symptoms at the subsequent assessment.
Discussion
This longitudinal study of recent interpersonal violence (IPV) survivors examined whether patterns of diurnal cortisol and alpha-amylase secretion were associated with concurrent PTSD diagnosis or symptom severity and predicted subsequent PTSD development. We also examined the independent effects of depressive symptoms and childhood trauma exposure on diurnal HPA/SNS secretion. Multilevel models revealed a pattern of lower waking cortisol levels and slower diurnal cortisol decline that distinguished women with concurrent PTSD from IPV survivors without PTSD and healthy non-traumatized controls (NTC). Moreover, lagged effects models demonstrated this diurnal cortisol secretion pattern predicted PTSD diagnostic status at the next assessment, which supports interpretation of this pattern as a risk marker for – rather than simply a consequence of – PTSD. Thus, a pattern of lower waking cortisol levels and flatter diurnal cortisol slope may serve as a predictive marker for early clinical course and may be important in PTSD prevention efforts. For example, if findings are replicated, it is possible that lower waking cortisol and flatter diurnal cortisol slope may be useful in identifying those who may be most likely to benefit from early, modified prolonged exposure therapy, which has shown promise for prevention of posttraumatic stress and depressive symptoms (Rothbaum et al., 2012).
Results support a growing body of literature suggesting a neurobiological pathway for the development of PTSD symptoms via hypothesized effects on trauma memory consolidation (de Quervain et al., 2009), context processing (Liberzon & Abelson, 2016), and circadian rhythm (Agorastos et al., 2020). Lower waking cortisol levels were associated with greater depressive symptoms. Moreover, lower waking cortisol levels and flatter diurnal cortisol slopes also characterized women with greater childhood trauma exposure. When simultaneously examining the effect of childhood trauma exposure, and depression, lower waking cortisol levels - but not flatter diurnal cortisol slopes - remained significantly associated with concurrent and subsequent PTSD diagnosis, while higher childhood trauma continued to predict flatter diurnal cortisol slopes. Notably, lower waking cortisol levels were also associated with higher mean posttraumatic stress symptom severity among IPV survivors. Together, these findings demonstrate distinct contributions of childhood trauma exposure and PTSD to diurnal cortisol secretory patterns and highlight the potential utility of blunted waking cortisol levels as a prognostic biomarker of PTSD risk and flatter diurnal cortisol slope as an index of childhood trauma among recent IPV survivors.
Exposure to past trauma is associated with altered HPA functioning, including flatter diurnal cortisol slopes compared to controls (Nicolson & Ponnamperuma, 2019; Thompson et al., 2015), and increased risk of PTSD onset following subsequent trauma exposures (Delahanty & Nugent, 2006). Consistent with prior research, we found evidence of flatter diurnal cortisol slopes in individuals with greater childhood trauma history and an association between lower waking cortisol and concurrent and subsequent PTSD diagnosis. Higher mean posttraumatic stress symptom severity was associated with lower waking cortisol levels among IPV survivors, consistent with prior work showing blunted cortisol awakening responses in individuals with PTSD compared to trauma-exposed and healthy individuals (Rauch et al., 2020; Wessa et al., 2006). These findings support theoretical models which posit that prior trauma exposures progressively attenuate cortisol secretion, thereby increasing risk for PTSD onset (Steudte-Schmiedgen et al., 2016).
Contrary to expectations, diurnal cortisol slopes were not significantly related to concurrent or subsequent PTSD diagnosis when simultaneously examining the effect of childhood trauma. One interpretation is that flatter diurnal cortisol slopes are more strongly tied to childhood trauma exposure than to PTSD diagnosis. Another possibility is that diurnal cortisol slopes are more closely related to the daily experiences of trauma survivors than to monthly variation in posttraumatic stress symptoms captured by the CAPS interview. Momentary changes in mood are known to influence daily fluctuations in cortisol levels (Smyth et al., 1998). Future studies are needed to capture daily covariation between diurnal cortisol rhythms and emotional state (Adam et al., 2006) among recent IPV survivors.
Regarding SNS activity, multilevel models revealed women with greater childhood trauma exposure displayed higher waking alpha-amylase levels and slower diurnal alpha-amylase increase compared to women with lower childhood trauma exposure. This is partly consistent with evidence of heightened SNS activity among individuals with PTSD compared to controls (Buckley & Kaloupek, 2001; O’Donnell et al., 2004; Pole, 2007), including elevated waking alpha-amylase levels (Keeshin et al., 2015). PTSD diagnostic status and depressive symptoms were not associated with alpha-amylase.
Notably, within-person changes in depressive symptoms from assessment to assessment were not associated with waking cortisol or diurnal cortisol slopes. While mean depressive symptoms (i.e., between-person differences in depressive symptoms) were associated with waking cortisol, the direction of the effect was counter to expectations; higher depressive symptoms were associated with lower waking cortisol. Furthermore, the relationship between depressive symptoms and waking cortisol was no longer significant when simultaneously examining PTSD diagnostic status. One possible explanation for these discrepant findings is that PTSD diagnosis better explains participants’ low mood, rather than major depression. Moreover, elevated cortisol levels are more likely to be found in melancholic than non-melancholic depression (Juruena et al., 2018). Individuals with melancholic depression are more likely to be found in inpatient, compared to outpatient, settings and are more likely to display severe depressive symptoms. In contrast, participants in the present sample were recruited from hospitals and community settings and displayed moderate depressive symptoms. Additionally, there was no relationship between waking alpha-amylase levels or diurnal alpha-amylase slopes and concurrent or subsequent PTSD diagnostic status or depressive symptoms. Future studies are needed to replicate these findings and to evaluate plausible mechanisms linking posttraumatic psychopathology to waking levels of cortisol. For example, research suggests that whereas increased engagement in daily social activities is associated with more pronounced, normative declines in cortisol levels for non-depressed individuals, daily social activities are uncoupled from diurnal cortisol secretion for depressed individuals (Stetler et al., 2004). It is unknown whether this pattern of uncoupling of daily social activities and decline in cortisol levels is present in individuals with PTSD.
Findings regarding diurnal neuroendocrine secretion in IPV survivors complement prior work examining changes in HPA/SNS stress response patterns following IPV exposure in an overlapping sample (Morris et al., 2020). Recent IPV survivors who develop PTSD are characterized by blunted diurnal cortisol secretion (i.e., lower waking cortisol levels, flatter diurnal cortisol slopes), heightened cortisol reactivity to initial presentation of a social-evaluative stress task compared to NTC, and rapid habituation of cortisol responses to subsequent stressors. In contrast, recent IPV survivors who do not develop PTSD exhibit steeper diurnal cortisol declines than those with PTSD and blunted cortisol reactivity to social-evaluative stressors compared to NTC. Although it is tempting to speculate that relatively preserved diurnal cortisol declines and blunted cortisol responses to social-evaluative threat together comprise an early biomarker of IPV resilience, these neuroendocrine features may yet confer increased vulnerability for developing PTSD in the context of future traumatic events (Steudte-Schmiedgen et al., 2016).
Limitations of the present study can guide future research on early predictors of post-IPV PTSD development. The rigorous saliva collection protocol for capturing within-person changes in diurnal HPA/SNS rhythms over time precluded a large sample size. Larger studies of IPV survivors are needed to identify diurnal neuroendocrine secretion components associated with membership in distinct posttraumatic and depressive symptom change trajectories. Additionally, the present study excluded male participants. Future research should examine whether gender moderates the relationship between diurnal cortisol and alpha-amylase secretion and symptomatology following IPV exposure. The ‘post-post’ longitudinal design cannot shed light on whether alterations in daily cortisol and alpha-amylase secretion were present before IPV exposure – a distinct possibility given results demonstrating a key role for childhood trauma exposure as a determinant of waking levels and diurnal slopes. Moreover, there is evidence for transgenerational transmission of PTSD vulnerability from mother to offspring. This is thought to occur through maternal epigenetic programming, which leads to lower cortisol levels in offspring (Yahyavi et al., 2014). Future research is needed to fully understand the relationship between generational and childhood trauma, cortisol secretion, and PTSD. Finally, sleep, circadian rhythm, and neuroendocrine markers are closely linked and all play a role in the neurobiology of PTSD (Agorastos et al., 2020). While participants were asked to self-report their sleep, the present study did not carefully investigate the role of sleep and circadian rhythm in participants’ clinical presentation. Future studies should include objective measures of sleep/wake cycles (e.g., actigraphy) to clarify the role of sleep and circadian rhythms in the development of PTSD symptoms. Nevertheless, the longitudinal design and lagged effects analytic approach were in line with recommendations for interpreting the causal direction of effects for diurnal cortisol secretion on health outcomes (Adam et al., 2017).
In summary, lower waking cortisol was associated with concurrent PTSD diagnostic status and symptom severity, as well as PTSD diagnosis at the subsequent assessment, while greater childhood trauma was associated with a slower diurnal cortisol decline, higher waking alpha-amylase, and slower diurnal alpha-amylase increase. Findings highlight the potential utility of blunted waking cortisol as an early biomarker of PTSD symptoms and risk of developing PTSD. Future studies employing daily diary or ecological momentary assessment paradigms are needed to examine how daily experiences shape the diurnal cortisol secretion patterns associated with risk for subsequent PTSD. This knowledge could inform early cognitive-behavioral interventions designed to promote resilience in the aftermath of IPV (Rothbaum et al., 2012). Childhood trauma was an important correlate of both HPA and SNS activity, with greater exposure associated with blunted diurnal cortisol and alpha-amylase slopes and higher waking alpha-amylase. Childhood trauma should be assessed in research and clinical care, as results suggest it is associated with the dynamics of stress response in those at risk for PTSD.
Supplementary Material
Acknowledgements
This work was supported, in part, by grants from the National Institutes of Health (K01 MH101403, U54 MD007593, U54MD007586, R01MH108155, R01MD010757 and R01DA040966). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additionally, the authors are grateful for the comments of Dr. Chrystyna Kouros and Dr. Lesa Hoffman.
Footnotes
Competing Interests
The authors report no conflicts of interest.
When controlling for self-reported amount of sleep in the combined model predicting concurrent diagnostic status, childhood trauma continued to predict diurnal cortisol slope (b=0.00002, SE=0.00001, p=.001). The relationship between PTSD diagnostic status and waking cortisol only approached significance (b=-0.1927, SE=0.1024, p=.061) when controlling for self-reported sleep.
When controlling for self-reported amount of sleep in the combined model predicting subsequent diagnostic status, the relationship between waking cortisol level and PTSD diagnostic status at the subsequent assessment remained significant (b=-0.6149, SE=0.1295, p<.001).
When controlling for self-reported amount of sleep in the combined model predicting concurrent PTSD symptom severity within the IPV group, within-person (i.e., between-assessment) PTSD symptom severity was not associated with waking cortisol (b=0.0052, SE=0.0044, p=.242), but between-person mean PTSD symptom severity remained a significant predictor of waking cortisol (b=-0.0176, SE=0.0069, p=.014).
When controlling for self-reported amount of sleep, the relationship between childhood trauma and waking alpha-amylase was no longer significant (b=-0.0083, SE=0.0057, p=.148), but childhood trauma continued to predict diurnal alpha-amylase slope (b=-0.00001, SE=0.00001, p=.005).
References
- Adam EK, Hawkley LC, Kudielka BM, & Cacioppo JT (2006). Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences of the United States of America, 103(45), 17058–17063. 10.1073/pnas.060503103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, & Gilbert KE (2017). Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology, 83, 25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agorastos A, Nicolaides NC, Bozikas VP, Chrousos GP, & Pervanidou P (2020). Multilevel Interactions of Stress and Circadian System: Implications for Traumatic Stress. Front Psychiatry, 10, 1003. 10.3389/fpsyt.2019.01003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, & Ranieri WF (1996). Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients [Article]. Journal of personality assessment, 67(3), 588–597. 10.1207/s15327752jpa6703_13 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological), 57(1), 289–300. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, & Ruggiero J (1994). Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry, 151(8), 1132–1136. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a clinician-administered PTSD scale. Journal of traumatic stress, 8(1), 75–90. 10.1002/jts.2490080106 [DOI] [PubMed] [Google Scholar]
- Booij SH, Bos EH, Bouwmans ME, van Faassen M, Kema IP, Oldehinkel AJ, & de Jonge P (2015). Cortisol and α-amylase secretion patterns between and within depressed and non-depressed individuals. PloS one, 10(7), e0131002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe T, Jacob J, Kröger C, & Walker J (2020). How expensive are post-traumatic stress disorders? Estimating incremental health care and economic costs on anonymised claims data. The European Journal of Health Economics, 21, 917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TC, & Kaloupek DG (2001). A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosomatic medicine, 63(4), 585–594. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ-F, Aerni A, Schelling G, & Roozendaal B (2009). Glucocorticoids and the regulation of memory in health and disease. Frontiers in neuroendocrinology, 30(3), 358–370. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, & Nugent NR (2006). Predicting PTSD prospectively based on prior trauma history and immediate biological responses. Annals of the New York Academy of Sciences, 1071(1), 27–40. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (2005). Structured clinical interview for DSM-IV-TR Axis I disorders: patient edition. Biometrics Research Department, Columbia University; New York, NY. [Google Scholar]
- Hall M, Vasko R, Buysse D, Ombao H, Chen Q, Cashmere JD, Kupfer D, & Thayer JF (2004). Acute stress affects heart rate variability during sleep. Psychosomatic medicine, 66(1), 56–62. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support [Article]. Journal of Biomedical Informatics, 42(2), 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juruena MF, Bocharova M, Agustini B, & Young AH (2018). Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. Journal of affective disorders, 233, 45–67. [DOI] [PubMed] [Google Scholar]
- Keeshin BR, Strawn JR, Out D, Granger DA, & Putnam FW (2015). Elevated salivary alpha amylase in adolescent sexual abuse survivors with posttraumatic stress disorder symptoms. Journal of child and adolescent psychopharmacology, 25(4), 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, & Friedman MJ (2013). National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. Journal of traumatic stress, 26(5), 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, & Hellhammer DH (1989). Salivary cortisol in psychobiological research: An overview. Neuropsychobiology, 22(3), 150–169. [DOI] [PubMed] [Google Scholar]
- Liberzon I, & Abelson JL (2016). Context processing and the neurobiology of post-traumatic stress disorder. Neuron, 92(1), 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse M-L, Reitsma JB, De Vries G-J, Gersons BP, & Olff M (2007). Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. The British Journal of Psychiatry, 191(5), 387–392. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans [Review]. Psychological Bulletin, 133(1), 25–45. 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Morris MC, Bailey B, Hellman N, Williams A, Lannon EW, Kutcher ME, Schumacher JA, & Rao U (2020). Dynamics and determinants of cortisol and alpha-amylase responses to repeated stressors in recent interpersonal trauma survivors. Psychoneuroendocrinology, 104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Compas BE, & Garber J (2012). Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clinical psychology review, 32(4), 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Hellman N, Abelson JL, & Rao U (2016). Cortisol, heart rate, and blood pressure as early markers of PTSD risk: A systematic review and meta-analysis. Clinical Psychology Review, 49, 79–91. 10.1016/j.cpr.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, & Rao U (2013). Psychobiology of PTSD in the acute aftermath of trauma: Integrating research on coping, HPA function and sympathetic nervous system activity. Asian Journal of Psychiatry, 6(1), 3–21. 10.1016/j.ajp.2012.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater UM, & Rohleder N (2009). Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology, 34(4), 486–496. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Schlotz W, Ehlert U, & Kirschbaum C (2007). Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology, 32(4), 392–401. [DOI] [PubMed] [Google Scholar]
- Nicholson EL, Bryant RA, & Felmingham KL (2014). Interaction of noradrenaline and cortisol predicts negative intrusive memories in posttraumatic stress disorder. Neurobiology of Learning and Memory, 112, 204–211. [DOI] [PubMed] [Google Scholar]
- Nicolson NA, & Ponnamperuma T (2019). Gender moderates diurnal cortisol in relation to trauma and PTSD symptoms: A study in Sri Lankan adolescents. Psychoneuroendocrinology, 104, 122–131. [DOI] [PubMed] [Google Scholar]
- O’Donnell T, Hegadoren KM, & Coupland N (2004). Noradrenergic mechanisms in the pathophysiology of post-traumatic stress disorder. Neuropsychobiology, 50(4), 273–283. [DOI] [PubMed] [Google Scholar]
- Pole N (2007). The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychological Bulletin, 133(5), 725–746. 10.1037/0033-2909.133.5.725 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2006). Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31(4), 437–448. 10.3102/10769986031004437 [DOI] [Google Scholar]
- Rauch SA, King A, Kim HM, Powell C, Rajaram N, Venners M, Simon NM, Hamner M, & Liberzon I (2020). Cortisol awakening response in PTSD treatment: Predictor or mechanism of change. Psychoneuroendocrinology, 118, 104714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, Cheong Y, Congdon R, & du Toit M (2019). HLM 8: Hierarchical linear and nonlinear modeling. Skokie, IL: Scientific Software International. [Google Scholar]
- Rothbaum BO, Kearns MC, Price M, Malcoun E, Davis M, Ressler KJ, Lang D, & Houry D (2012). Early intervention may prevent the development of posttraumatic stress disorder: a randomized pilot civilian study with modified prolonged exposure. Biol Psychiatry, 72(11), 957–963. 10.1016/j.biopsych.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Keinan G, & Daon K (2004). Effects of stress on sleep: the moderating role of coping style. Health Psychology, 23(5), 542. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Gevonden M, Ratanatharathorn A, Laska E, Van Der Mei WF, Qi W, Lowe S, Lai BS, Bryant RA, & Delahanty D (2019). Estimating the risk of PTSD in recent trauma survivors: results of the International Consortium to Predict PTSD (ICPP). World Psychiatry, 18(1), 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, & Essex MJ (2008). Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 50(7), 690–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijbrandij M, Kleiboer A, Bisson JI, Barbui C, & Cuijpers P (2015). Pharmacological prevention of post-traumatic stress disorder and acute stress disorder: a systematic review and meta-analysis [Article]. Lancet Psychiatry, 2(5), 413–421. 10.1016/s2215-0366(14)00121-7 [DOI] [PubMed] [Google Scholar]
- Smyth J, Ockenfels MC, Porter L, Kirschbaum C, Hellhammer DH, & Stone AA (1998). Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology, 23(4), 353–370. [DOI] [PubMed] [Google Scholar]
- Speer KE, Semple S, Naumovski N, D’Cunha NM, & McKune AJ (2019). HPA axis function and diurnal cortisol in post-traumatic stress disorder: A systematic review. Neurobiology of stress, 11, 100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler C, Dickerson SS, & Miller GE (2004). Uncoupling of social zeitgebers and diurnal cortisol secretion in clinical depression. Psychoneuroendocrinology, 29(10), 1250–1259. [DOI] [PubMed] [Google Scholar]
- Steudte-Schmiedgen S, Kirschbaum C, Alexander N, & Stalder T (2016). An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: Insight from recent hair cortisol findings. Neuroscience and Biobehavioral Reviews, 69, 124–135. 10.1016/j.neubiorev.2016.07.015 [DOI] [PubMed] [Google Scholar]
- Thoma MV, Joksimovic L, Kirschbaum C, Wolf JM, & Rohleder N (2012). Altered salivary alpha-amylase awakening response in Bosnian War refugees with posttraumatic stress disorder. Psychoneuroendocrinology, 37(6), 810–817. [DOI] [PubMed] [Google Scholar]
- Thompson DJ, Weissbecker I, Cash E, Simpson DM, Daup M, & Sephton SE (2015). Stress and cortisol in disaster evacuees: an exploratory study on associations with social protective factors. Applied psychophysiology and biofeedback, 40(1), 33–44. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Weibel L, Spiegel K, Leproult R, Dugovic C, & Maccari S (2000). Interactions between stress and sleep: from basic research to clinical situations. Sleep medicine reviews, 4(2), 201–220. [Google Scholar]
- Weathers FW, Ruscio AM, & Keane TM (1999). Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychological assessment, 11(2), 124. [Google Scholar]
- Wessa M, Rohleder N, Kirschbaum C, & Flor H (2006). Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology, 31(2), 209–215. 10.1016/j.psyneuen.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Yahyavi ST, Zarghami M, & Marwah U (2014). A review on the evidence of transgenerational transmission of posttraumatic stress disorder vulnerability. Brazilian Journal of Psychiatry, 36(1), 89–94. [DOI] [PubMed] [Google Scholar]
- Yehuda R (2002). Clinical relevance of biologic findings in PTSD. Psychiatric Quarterly, 73(2), 123–133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


