Figure 9.
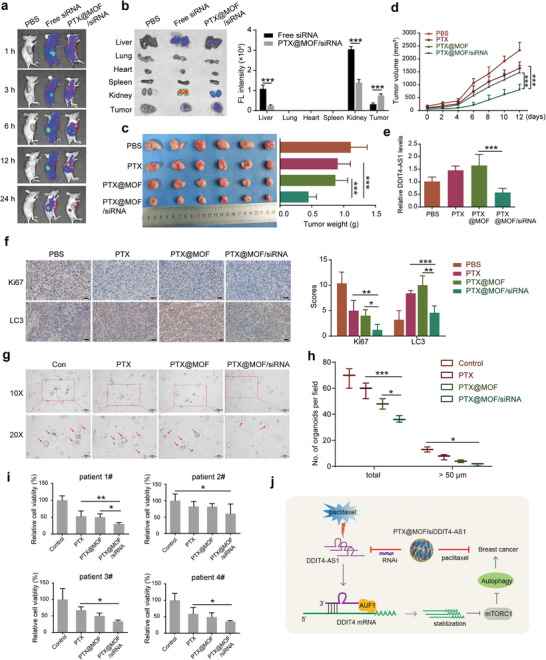
In vivo biodistribution and antitumor activity of the codelivery nanoparticles. a) In vivo fluorescence images of mice were taken at indicated time points postinjection. b) Representative ex vivo fluorescence images and quantitative fluorescence intensity of tumors and organs collected from the mice at 24 h postinjection (n = 3 mice per group, ***p < 0.001). c) Image of collected tumors and tumor weight of the MDA‐MB‐231 tumor‐bearing mice treated with the formulas. ***p < 0.001. d) Tumor growth curves of mice after different treatments. ***p < 0.001. e) The quantified DDIT4‐AS1 levels after different treatments by qRT‐PCR analysis. ***p < 0.001. f) Representative Ki67 and LC3 staining and quantitative analysis of orthotopic xenograft sections from different groups. Scale bar, 200 µm. *p < 0.05, **p < 0.01, ***p < 0.001. g,h) Bright‐field images depicting patient‐derived organoid with indicated treatments and number of organoids per field were calculated. *p < 0.05, ***p < 0.001. i) Cell viability was detected using different breast cancer patient‐derived organoids. *p < 0.05, **p < 0.01. j) Schematic of the proposed mechanism of DDIT4‐AS1 in TNBC. Mechanistically, cytosolically localized DDIT4‐AS1 could stabilize DDIT4 mRNA via recruiting the RNA binding protein AUF1 and promoting the interaction between DDIT4 mRNA and AUF1, which subsequently inactivates mTORC1, activates autophagy and promotes breast cancer progression. Besides, the treatment of chemotherapeutic agent paclitaxel (PTX) could induce upregulation of DDIT4‐AS1 and further lead to cyto‐protective autophagy in TNBC, limiting the tumor killing effect. Therefore, a smart core–shell metal–organic framework (MOF) nanosystem was fabricated to effectively load DDIT4‐AS1 siRNA and PTX, providing opportunities for combined gene‐drug TNBC therapy.
