FIGURE 1.
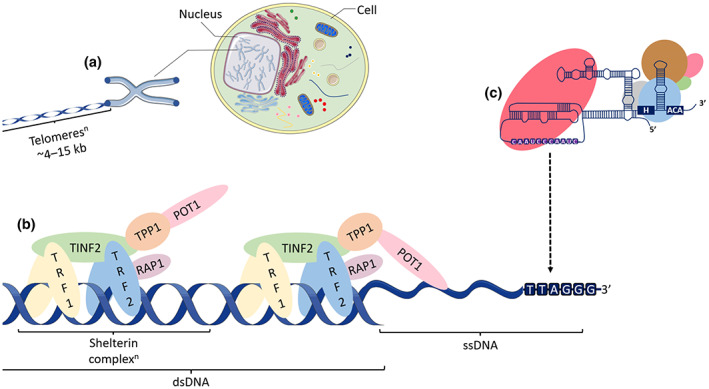
Telomeres, shelterin and the canonical function of telomerase. (a) Telomeres are a repetitive sequence of DNA (5′‐TTAGGGn‐3′) that cap the ends of chromosomes and preserve genomic integrity. Average telomere length in humans is typically 4–15 kilobases (kb) long. Given that telomerase is expressed at very low levels in healthy human cells, telomeres shorten with successive cell divisions due to the end replication problem and DNA damage. (b) Shelterin is comprised of six telomere‐repeat binding proteins (telomere repeat binding factor 1 [TRF1], telomeric repeat binding factor 2 [TRF2], TRF1 interacting nuclear factor 2 [TINF2], TRF2 interacting protein [RAP1], ACD shelterin complex subunit and telomerase recruitment factor [TPP1], and protection of telomeres 1 [POT1]) that bind telomeric DNA directly (protein homodimers, TRF1/TRF2) or indirectly (TINF2, TPP1 and RAP1) via TRF1/2. POT1 preferentially binds to single‐stranded telomeric DNA (G‐overhang). Note that this is the open telomere state, as shelterin are responsible for directing other structures at telomeres (closed states) (e.g. T‐ and D‐loops and other formations) (Lim & Cech, 2021; Zinder et al., 2022). (c) Human telomerase is comprised of a catalytic core ribonucleoprotein (RNP) (telomerase reverse transcriptase [TERT] protein and telomerase RNA component [TERC]) and an H/ACA RNP complex with H/ACA ribonucleoproteins: dyskerin (blue), NOP10 (green), NHP2 (pink), GAR1 (grey) and TCAB1 (brown) (Liu et al., 2022; Nguyen et al., 2018). The template region of telomerase interacts with the g‐strand telomeric DNA via the complementary TERC sequence (white RNA with purple background) for telomere synthesis. TERT is indicated by the light red oval. Supported by Servier Medical Art.
