FIGURE 2.
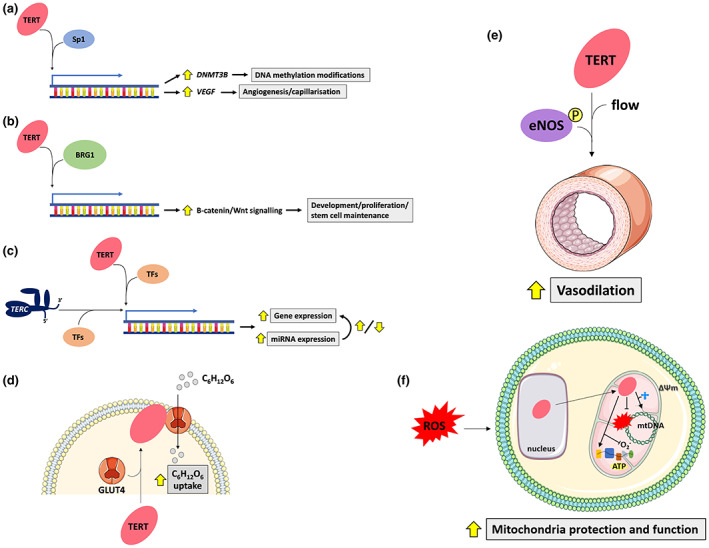
Summary of the key non‐canonical functions of telomerase relevant to endurance training adaptations. (a) TERT binds to Sp1 and transcribes its target genes DNMT3B (Yu et al., 2018) and VEGF (Liu et al., 2016) thereby contributing to de novo DNA methylation changes and angiogenesis, respectively. This appears to be a telomere length‐independent non‐canonical function of TERT, as catalytically inert TERT exerts identical functions (Liu et al., 2016). (b) TERT interacts with BRG1, which targets genes involved in the β‐catenin/Wnt signalling pathway to regulate development and stem cell maintenance. (c) TERT and TERC appear to independently regulate gene and microRNA (miRNA) expression likely through cooperation with other transcription factors (TFs) to controls genes (e.g. those regulating cell senescence). (d) TERT is shuttled to the plasma membrane upon insulin treatment. There, it colocalises with glucose (C6H12O6) transporters (1, 4 and 12) and increases glucose uptake in skeletal muscle (Shaheen et al., 2014). (e) Increased TERT expression via its transcriptional activator, AGS‐499, increases flow‐mediated vasodilation in HUVECs from coronary artery disease patients via enhancing endothelial nitric oxide synthase (eNOS) signalling (Beyer et al., 2016). (f) TERT is shuttled from the nucleus into the mitochondria in the presence of elevated reactive oxygen species (ROS). Inside the mitochondria, TERT improves mitochondrial function and protects mitochondrial DNA (mtDNA) from ROS‐induced damage. It also acts as a reverse transcriptase with mitochondrial transfer RNAs, increases oxygen flux in the electron transport chain and enhances membrane potential (∆Ψ m). Supported by Servier Medical Art.
