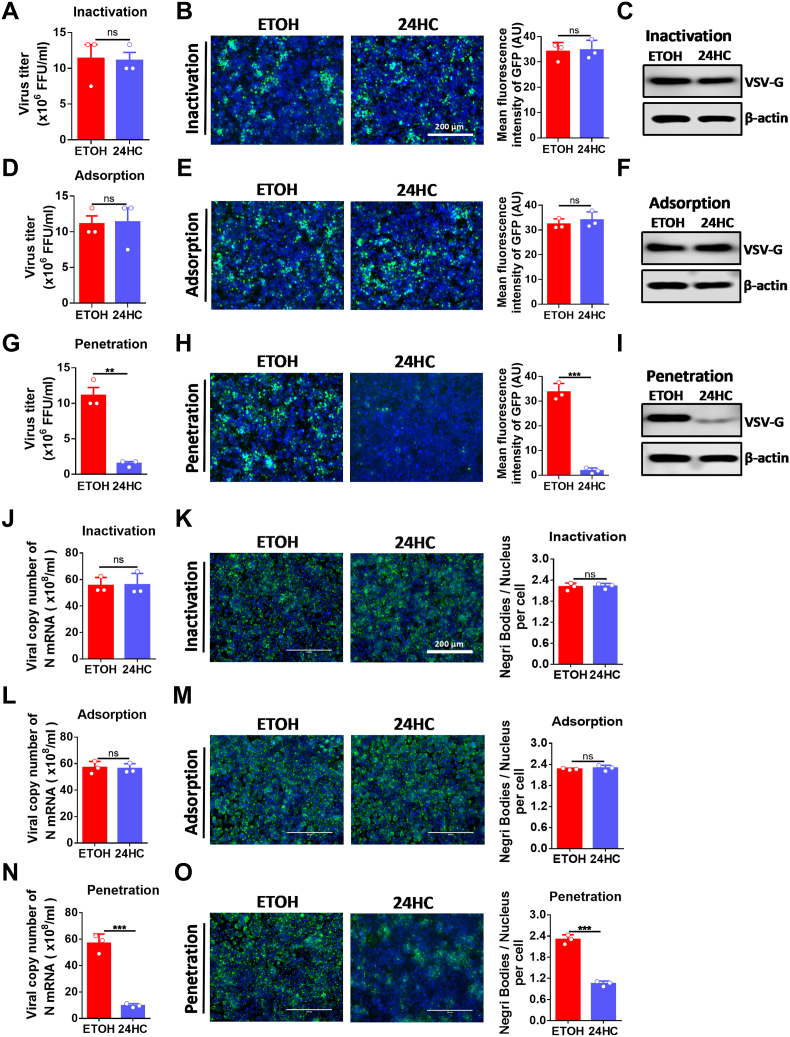
Fig. 4.
24HC exerts its antiviral function in the step of virus penetration. A-C. VSV inactivation assay. VSV-GFP (MOI = 0.01) and 24HC (5 μM) or ETOH were incubated together at 37 °C for 3 h, N2a cells cultured in 24-well plates were prechilled at 4 °C for 1 h, then the media were replaced by the mixture of 24HC or ETOH and VSV-GFP. After incubation at 4 °C for another 2 h, cells were washed and incubated at 37 °C for 12 h in a medium devoid of 24HC, and cell supernatants were collected for virus titration (n = 3) (A). Cells were fixed for detection of GFP fluorescence signals by fluorescence microscope. Mean fluorescence intensities of GFP were calculated by ImageJ software and were placed on the right side of corresponding fluorescence images (n = 3) (B), scale bar = 200 μm. Cells under the same treatment were collected for WB analysis (C). D-F. VSV adsorption assay. N2a cells cultured in 24-well plates were prechilled at 4 °C for 1 h, and then the media were replaced by a mixture of 24HC (5 μM) or ETOH and VSV-GFP (MOI = 0.01). After incubation at 4 °C for another 2 h, cells were washed and incubated at 37 °C for 12 h in a medium devoid of 24HC, and cell supernatants were collected for virus titration (n = 3) (D). Cells were fixed and observed by fluorescence microscopy (n = 3) (E), scale bar = 200 μm. Cells under the same treatment were collected for WB (F). G-I. VSV penetration assay. N2a cells cultured in 24-well plates were prechilled at 4 °C for 1 h and then incubated with VSV-GFP (MOI = 0.01) for 2 h at 4 °C. The virus-containing medium was replaced with fresh medium containing 24HC (5 μM) or ETOH, and the temperature was shifted to 37 °C for 3 h. Cells were washed and incubated at 37 °C for another 9 h in a medium devoid of 24HC, and cell supernatants were collected for virus titration (n = 3) (G). Cells were fixed and observed by fluorescence microscopy (n = 3) (H), scale bar = 200 μm. Cells under the same treatment were collected for WB (I). J, K. RABV inactivation assay. RABV (MOI = 0.01) and 24HC (5 μM) or ETOH were incubated together at 37 °C for 3 h, N2a cells cultured in 24-well plates were prechilled at 4 °C for 1 h, then the media were replaced with the mixture of 24HC or ETOH and RABV. After incubation at 4 °C for another 2 h, the cells were washed and incubated at 37 °C. After 48 h of incubation in a medium devoid of 24HC, cells were harvested to detect RABV nucleoprotein (RABV-N) mRNA levels by qPCR (n = 3) (J), or fixed and stained with anti-RABV N antibody to detect Negri bodies (RABV viral factories) and nucleus (n = 3) (K). The fluorescence intensity of Negri bodies/Nucleus was calculated using ImageJ software and placed on the right side of the corresponding fluorescence images, scale bar = 200 μm. L, M. RABV adsorption assay. N2a cells cultured in 24-well plates were prechilled at 4 °C for 1 h, and then the media were replaced with a mixture of 24HC (5 μM) or ETOH and RABV (MOI = 0.01). After incubation at 4 °C for another 2 h, the cells were washed and incubated at 37 °C. After 48 h of incubation in a medium devoid of 24HC, cells were harvested to detect RABV Nucleoprotein mRNA levels using qPCR (n = 3) (L), or fixed and stained to detect Negri bodies and nucleus (n = 3) (M), scale bar = 200 μm. N, O. RABV penetration assay. N2a cells cultured in 24-well plates were prechilled at 4 °C for 1 h and then incubated with RABV (MOI = 0.01) at 4 °C for 2 h. The virus-containing medium was replaced with fresh medium containing 24HC (5 μM) or ETOH, and the temperature was shifted to 37 °C for 3 h. The cells were washed and incubated at 37 °C. After 48 h of incubation in a medium devoid of 24HC, cells were harvested to detect RABV nucleoprotein mRNA levels by qPCR (n = 3) (N), or fixed and stained to detect Negri bodies and nucleus (n = 3) (O), scale bar = 200 μm. Statistical analysis of comparisons between groups was performed by Student's t-test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant). Bar graph shows mean ± SD. Western blot data are representative of at least two independent experiments.