Significance
The FKBP5 gene is a key genetic risk factor for stress-related disorders. The highly divergent symptomology of psychiatric disorders highlights that genetic risk factors like FKBP5 do not simply affect stress-coping mechanisms in a unilateral manner. Rather, we here demonstrate that FKBP5 affects cognitive and emotional behavior, brain structure, and brain region-specific gene expression profiles in a highly cell-type and sex-specific manner. Our results underline that labelling a stress—sensitive factor as FKBP5 as “risk factor” is too simplistic, as loss of FKBP5 can have opposite effects on brain and behavior, dependent on which cell type and sex the loss is taking place. The results are of high importance for the development of targeted intervention strategies for psychiatric disorders.
Keywords: FKBP51, sex differences, aging, stress-related disorders, behavior
Abstract
Mental health disorders often arise as a combination of environmental and genetic factors. The FKBP5 gene, encoding the GR co-chaperone FKBP51, has been uncovered as a key genetic risk factor for stress-related illness. However, the exact cell type and region-specific mechanisms by which FKBP51 contributes to stress resilience or susceptibility processes remain to be unravelled. FKBP51 functionality is known to interact with the environmental risk factors age and sex, but so far data on behavioral, structural, and molecular consequences of these interactions are still largely unknown. Here we report the cell type- and sex-specific contribution of FKBP51 to stress susceptibility and resilience mechanisms under the high-risk environmental conditions of an older age, by using two conditional knockout models within glutamatergic (Fkbp5Nex) and GABAergic (Fkbp5Dlx) neurons of the forebrain. Specific manipulation of Fkbp51 in these two cell types led to opposing effects on behavior, brain structure and gene expression profiles in a highly sex-dependent fashion. The results emphasize the role of FKBP51 as a key player in stress-related illness and the need for more targeted and sex-specific treatment strategies.
To cope well with the physical and psychological stressors that we are exposed to throughout our life span, an adequate response to stress is required. Insufficient coping may result in the development of stress-related disorders, such as depressive or anxiety disorders (1–4), which are one of the most pressing and costly burdens of modern society (5–8). In the past decades, it has become evident that mental health problems often arise as a combination of environmental and genetic factors (9–12) and genome wide association studies identified risk genes that play a role in psychiatric disorders (13–15). One gene that has been uncovered as a key genetic risk factor for stress-related illness is FKBP5 (16). As a co-chaperone to the glucocorticoid receptor (GR) directly affecting its sensitivity to circulating glucocorticoids (17), a central function of the encoded FKBP51 protein is the regulation of stress system activity (18–21). Emphasizing this central role, single nucleotide polymorphisms (SNPs) in the FKBP5 gene modulate the risk to psychiatric disease development in interaction with (early) environmental stress exposure (13, 22, 23). Moreover, pharmacological modulation of FKBP51 or genetic manipulation of Fkbp5 in rodents has already demonstrated its implications in stress resilience mechanisms (19, 20, 24–27). However, the contribution of FKBP51 to stress resilience processes may vary largely between different brain regions, or even so, between specific cell types, which has already been highlighted by previous work from our group and others (25–31).
Fkbp5 is expressed widely throughout the brain with especially high baseline expression levels in the hippocampus (32). The hippocampus is a brain region that is particularly sensitive to the effects of stress (33, 34), and it has been extensively implicated in the pathophysiology of major depressive disorder (MDD) (35–39). Glutamatergic neurons make up the vast majority of the highly divergent cell type profile of the hippocampus, which further comprises GABAergic interneurons, different glial and vascular cell populations (40, 41). The hippocampus is known to have a differential functionality along its longitudinal axis, in which the dorsal hippocampus (DHC) is mostly involved in (spatial) memory and learning processes, whereas the ventral hippocampus (VHC) is particularly involved in emotional regulation (42, 43). Furthermore, a region that has largely been associated with sustained fear and anxiety states is the bed nucleus of the striatum terminalis (BNST) (44, 45). In contrast to the hippocampus, the BNST is a structure that is particularly rich in GABAergic neurons (45) and a previous study from our group already demonstrated the presence of Fkbp5 messenger RNA (mRNA) within these GABAergic neurons, specifically in the oval BNST (ovBNST) (27).
Apart from genetic predispositions, there are also a number of other factors that contribute to the development of stress-related pathology. One of these contributing factors is age. Elderly people that suffer from MDD have increased treatment resistance and depressive symptoms worsen over the years, which is a predictor for deteriorating disability amongst the elderly population (46–48). Interestingly, with age, FKBP51 imposes a higher risk on developing stress-related illness. Data from both human and rodent studies demonstrated that epigenetic mechanisms cause an age-dependent elevated Fkbp5 induction (49–52), resulting in augmented intracellular FKBP51 levels, similar to what has been observed in individuals carrying the FKBP5 psychiatric risk allele (23). Adding to this, knockout (KO) of Fkbp5 in mice had an accumulating antidepressant effect across the lifespan (50). In addition to age, sex heavily impacts stress vulnerability and its associated diseases. Stress-related disorders such as MDD or anxiety disorders are about twice as common in women as in men (53–56). Although research from the past decades increasingly shed a light on the highly sex-dependent stress coping mechanisms (57–64), research in female subjects is still largely underrepresented. Even more so, data on the interaction between sex, age, and genetic risk factors in the context of stress-related disorders is extremely limited.
To tackle this scarcity in information, this study aimed to investigate the region, cell type- and sex-specific contribution of FKBP51 to stress vulnerability and resilience mechanisms under the high-risk environmental conditions of an older age. Using two different conditional KO models, that lack FKBP51 in either glutamatergic or GABAergic neurons of the forebrain, we demonstrated that this psychiatric risk factor affects behavior, brain structure and gene expression in a highly sex-dependent and cell type-divergent manner.
Results
Validation of Fkbp5 KO in Glutamatergic and GABAergic Neurons of the Forebrain and its Physiological Consequences in Male and Female Older Aged Mice.
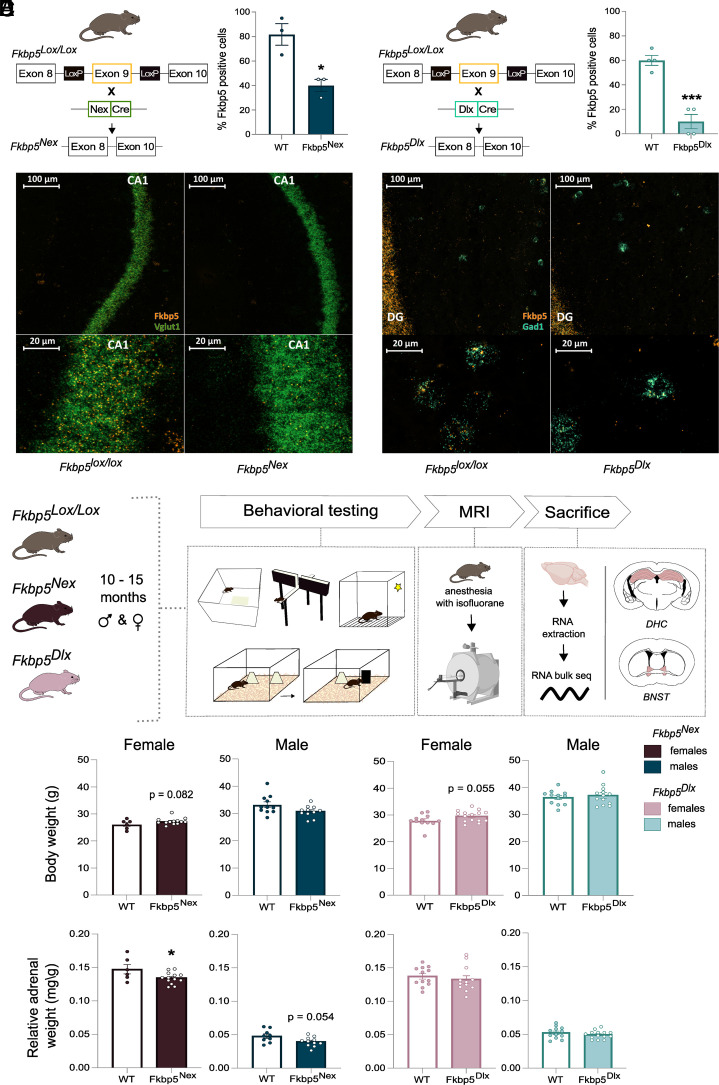
Fkbp5lox/lox mice were bred with Nex-Cre mice in order to achieve KO of Fkbp5 in glutamatergic neurons of the forebrain (Fig. 1A) (65, 66) or bred with Dlx5/6-Cre mice to induce loss of FKBP51 in GABAergic forebrain neurons (Fig. 1B) (67). Two separate RNAscope experiments were performed for Fkbp5, with well-known markers of glutamatergic and GABAergic neurons (Vglut1 and Gad1), to validate the successful KO of Fkbp5 mRNA in the cortex and DHC of glutamatergic and GABAergic neurons, respectively. The quantification of the RNAscope confocal images showed a significant reduction in the percentage of Fkbp5 positive cells within Vglut1 positive cells in Fkbp5Nex mice compared to Fkbp5lox/lox controls in cortical regions [t(4) = 4.11, P < 0.05; Fig. 1A]. Fkbp5 positive cells within Gad1 positive cells in Fkbp5Dlx mice and Fkbp5lox/lox controls revealed a significant reduction of Fkbp5 within GABAergic neurons of the DHC [t(6) = 7.07, P < 0.001; Fig. 1B]. As the RNAscope Fkbp5 probe targets the whole Fkbp5 mRNA and not only the deleted exon 9, the residual Fkbp5 signals in both lines in the targeted cell populations likely detect truncated mRNA that does not result in a functional FKBP51 protein. Representative confocal images of the dorsal CA1 of Fkbp5lox/lox and Fkbp5Nex mice and of the DHC for Fkbp5lox/lox and Fkbp5Dlx mice are illustrated (Fig. 1 A and B). Fkbp5 mRNA levels in off-target cell types for Fkbp5Nex and Fkbp5Dlx mice did not differ from Fkbp5lox/lox mice [Fkbp5Nex: t(4) = 1.0, P > 0.05; Fkbp5Dlx: t(4) = 0.173, P > 0.05; SI Appendix, Fig. S1].
Fig. 1.
Experimental setup and physiological measurements. By breeding Fkbp5lox/lox mice, in which exon 9 of the Fkbp5 gene is flanked by two loxP sites, with (A) Nex-Cre mice, a significant reduction of Fkbp5 mRNA in glutamatergic forebrain neurons was achieved. (B) Breeding of Fkbp5lox/lox mice with Dlx5/6-Cre mice resulted in a significant loss of Fkbp5 mRNA in GABAergic forebrain neurons. RNA scope confocal images of the DHC illustrated these significant reductions of Fkbp5 (orange panel) in glutamatergic CA1 neurons (Vglut1; green panel) and GABAergic interneurons (Gad1; blue panel). Ten- to fifteen-month–old Fkbp5Nex and Fkbp5Dlx mice of both sexes were exposed to a behavioral paradigm including tasks for anxiety, neutral and stressed cognition, followed by an MRI scanning procedure (C). After completion of the experiments, mice were sacrificed and tissue from the DHC and the BNST was collected for RNA bulk sequencing purposes. Analyses of physiological parameters revealed sex specific effects for body weight (D) and cell dependent changes in relative adrenal weight (E). Error bars represent mean ± SEM. *P <0.05, ***P < 0.001.
We then further investigated the functional, structural, and molecular consequences of the loss of Fkbp5 in these two distinct neuronal cell populations in the context of sex and older age. To this end, cohorts of older aged male and female Fkbp5Nex, Fkbp5Dlx and their wildtype (WT) Fkbp5lox/lox litter mates were generated and tested on different modalities (experimental timeline in Fig. 1C). Interestingly, physiological features were afflicted in a sex- or in a FKBP51-manipulated cell type-dependent manner. Body weight at baseline was only affected in female mice, in which Fkbp5Nex mice [F(1, 18) = 3.393, P = 0.082] and Fkbp5Dlx mice [t(25) = −2.01, P = 0.055] showed a trend towards an increased body weight, as compared to their Fkbp5lox/lox controls (Fig. 1D). However, relative adrenal weight was affected in a FKBP51-manipulated cell type-specific manner, where both Fkbp5Nex males [F(1, 18) = 4.24, P = 0.054] and females [F(1, 16) = 4.86, P < 0.05] had a reduced relative adrenal weight as compared to their WT control group. Adrenal weight of Fkbp5Dlx mice remained unaffected.
Loss of FKBP51 in Glutamatergic and GABAergic Neurons Leads to Differential and Sex-Dependent Effects on Behavior.
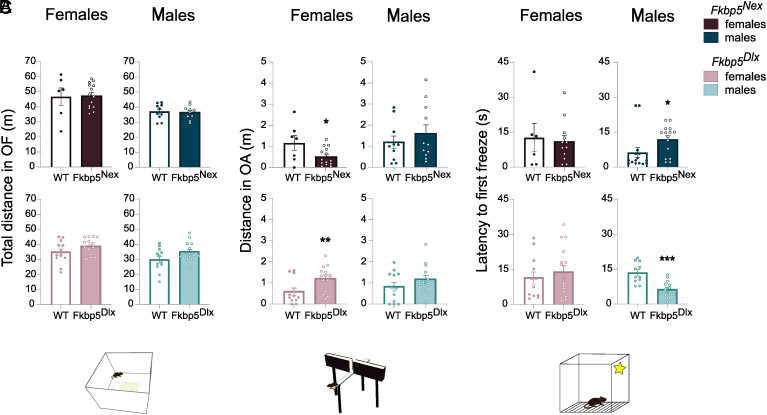
In addition to the physiological consequences of loss of FKBP51, we explored the effects of reduced FKBP51 on several behavioral parameters. First, effects of loss of FKBP51 on general locomotor behavior were excluded as measured by the total distance travelled in the open field (OF) (Fig. 2A). Interestingly, we did observe a sex-specific effect on anxiety-like behavior, in which female mice were solely affected (Fig. 2B). Moreover, apart from the sex-specific nature of the effect, the directionality of the effect on anxiety behavior was also dependent on the cell-type in which loss of FKBP51 took place. Lack of FKBP51 led to opposing effects on anxiety-like behavior depending on the cell type involved. A reduction of FKBP51 in glutamatergic neurons induced an increased anxiety-like behavior as measured by open arm distance in the elevated plus maze (EPM) [F(1, 18) = 5.44, P < 0.05], whereas mice that lack FKBP51 in GABAergic neurons showed an anxiolytic phenotype [t(25) = −2.77, P < 0.05].
Fig. 2.
Loss of Fkbp5 leads to cell- and sex-specific effects on behavior that are unique to certain behavior domains. No significant changes in locomotion (A) as measured by a 15-min Open Field test could be observed as a result of loss of Fkbp5 in older aged mice. However, loss of Fkbp5 in glutamatergic neurons leads to (B) an anxiety-like phenotype on the EPM, where loss in GABAergic neurons leads to anxiolytic behavior. These effects on anxiety are solely observed in female mice. On the contrary, cognitive behavior under stressful conditions is mainly affected in older aged male mice (C). Where loss of Fkbp5 in glutamatergic neurons reduces the memory of an adverse environment, a reduction in Fkbp5 in GABAergic neurons enhances the memory of an aversive event. Error bars represent mean ± SEM.*P < 0.05, **P <0.01, ***P < 0.001.
Furthermore, cognitive behavior was assessed using the novel object recognition (NOR) and spatial object recognition (SOR) task, testing memory performance in a neutral environment, and using the conditioned context retrieval for memory performance under stressful conditions. The loss of FKBP51 did not lead to any changes on cognitive behavior in a neutral context (SI Appendix, Fig. S2). Remarkably though, again a sex- and cell type-specific effect was observed for cognitive functioning in a stressful environment. In contrast to anxiety-like behaviors, changes in memory performance could only be observed in male mice (Fig. 2C). Fkbp5Nex male mice took a longer time to show freezing behavior in a familiar aversive environment than their Fkbp5lox/lox littermates, indicating a worsened memory of the aversive spatial context [F(1, 17) = 6.10, P < 0.05]. Fkbp5Dlx mice, on the other hand, displayed a more rapid freezing response as compared to their WT controls upon exposure to the aversive context [F(1, 22) = 20.97, P < 0.01], indicating an enhanced memory of the aversive location. To further emphasize the sex-dependent nature of the effects on physiological and behavioral parameters, additional information on main effects of sex and sex × genotype interactions can be found in SI Appendix, Table S1.
Loss of FKBP51 in Glutamatergic and GABAergic Neurons Leads to Pronounced Structural Brain Changes in Fkbp5Dlx Mice.
To investigate whether the observed sex- and cell-type–dependent behavioral changes upon loss of FKBP51 in either glutamatergic or GABAergic neurons are accompanied by structural brain differences, mice underwent a MRI scan succeeding the behavioral protocol.
Fkbp5Dlx vs. Fkbp5lox/lox.
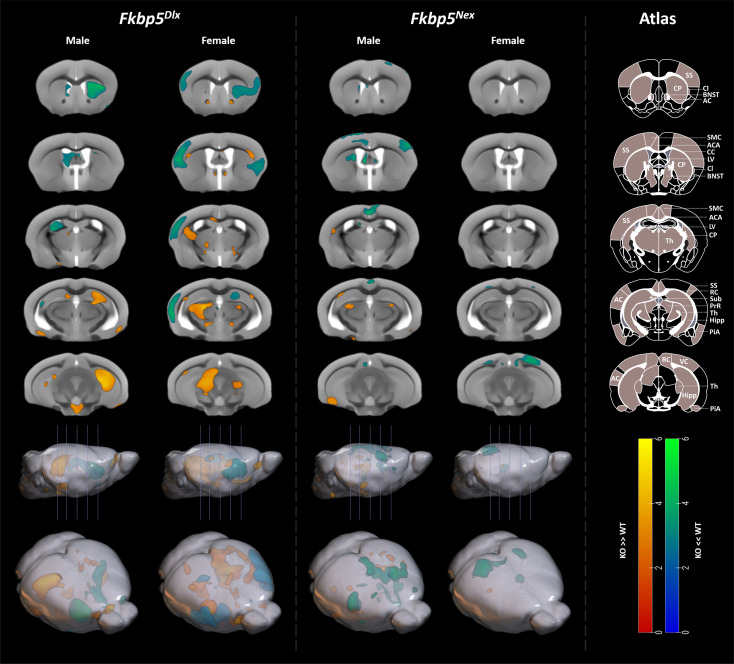
A two-way ANOVA of the deformation-based morphometry analyses revealed a main effect of genotype with strongest volumetric differences in the caudoputamen, thalamus, and hippocampus, but was otherwise restricted to white matter (WM) and cerebrospinal fluid regions (SI Appendix, Fig. S3). Interaction effects (genotype × sex) revealed clusters in the right DHC, and in the left primary and supplemental somatosensory areas (pFWE,cluster < 0.005, SI Appendix, Fig. S3). In addition, the left DHC and the right piriform area showed an interaction effect, although not surviving cluster FWE correction. These regions show higher volume differences in male mice (Fkbp5Dlx > Fkbp5lox/lox) than in female animals (SI Appendix, Fig. S3). The inverse interaction (female Fkbp5Dlx > Fkbp5lox/lox larger than male Fkbp5Dlx > Fkbp5lox/lox) showed clusters in the left pretectal region (pFWE,cluster = 0.025), and left caudoputamen, right pretectal region, right inferior colliculus and left ventral subiculum (no cluster correction, SI Appendix, Fig. S3). For male Fkbp5Dlx mice, post hoc analysis for genotype effects showed larger volumes in the right hippocampus (pFWE, cluster = 0.002) compared to Fkbp5lox/lox, with similar, though smaller, effects in the left hippocampus (Fig. 3). Fkbp5Dlx also had larger volumes in the hypothalamus; however, these clusters were only significant at an uncorrected threshold (puncorr < 0.001). Smaller volumetric differences at the border of the brain volume were not considered, as they most likely stem from individual differences during the digital brain extraction step. For the contrast Fkbp5Dlx < Fkbp5lox/lox, significant clusters were found in the right caudoputamen (pFWE,cluster = 0.029) and the left lateral ventricle (pFWE,cluster = 0.029; Fig. 3). Further underlying sex differences, brain volume was differentially affected by genotype in female mice as compared to male mice. For female mice, two clusters in the thalamus were found for the contrast Fkbp5Dlx > Fkbp5lox/lox [pFWE,cluster < 0.001 (left thalamus) and pFWE,cluster = 0.07 (right thalamus)]. Furthermore, a strongly significant grey matter (GM) cluster in the somatosensory cortex, claustrum and left auditory cortex was found for the cluster Fkbp5Dlx < Fkbp5lox/lox (pFEW,cluster < 0.001). In addition, several Fkbp5Dlx > Fkbp5lox/lox clusters in the WM (e.g., corpus callosum, anterior commissure) and clusters in GM areas surrounding the BNST and the nucleus accumbens regions occurred, although these did not survive clustfewFWE correction (Fig. 3).
Fig. 3.
Loss of Fkbp5 leads to the largest structural changes when restricted to GABAergic neurons of the forebrain. Data from the T2*-weighted MRI scan revealed underlying structural consequences of loss of Fkbp5 in either glutamatergic or GABAergic neurons of the forebrain, with the largest and most significant changes in Fkbp5Dlx male and female mice. For Fkbp5Dlx male mice, large and strongly significant increases in GM volume were found in the bilateral hippocampus compared to Fkbp5lox/lox controls, whereas GM volumes were significantly decreased in the caudoputamen and lateral ventricle. Female Fkbp5Dlx mice had larger GM volumes in the thalamus than their Fkbp5lox/lox controls and strongly significant smaller GM volumes in the somatosensory cortex, claustrum, and auditory cortex. In addition, a number of WM structures were altered such as the corpus callosum and some GM areas around the BNST, although these clusters did not survive FWE correction. Furthermore, Fkbp5Nex male mice had significantly smaller GM volumes in bilateral regions of the motor cortex extending to the anterior cingulate area vs. Fkbp5lox/lox controls and uncorrected increased GM volumes were found in the left caudoputamen, right piriform area, bilateral dorsal thalamus and left ventral subiculum. Female Fkbp5Nex mice had decreased GM volumes in the right anteriormedial visual area. Scales represent Z-scores. Yellow-red scale: KO > WT, Greenblue scale: KO < WT. AC = Auditory Cortex, ACA = Anterior Cingulate Area, CC = Corpus Callosum, Cl = Claustrum, CP = Caudoputamen, Hipp = Hippocampus, LV = Lateral Ventricle, PiA = Piriform Area, PrR = Pretectal region, RC = Retrosplenial Cortex, SMC = Sensorimotor cortex, SS = Somatosensory Cortex, Sub = Subiculum, Th = Thalamus, VC = Visual Cortex.
Fkbp5Nex vs. Fkbp5lox/lox.
Main effect of genotype showed nominal volumetric differences in the left caudoputamen, bilateral regions of the thalamus-related to both the polymodal association cortex and to the sensory motor cortex, and in retrosplenial and visual cortical areas, however, not survfewng FWE correction (SI Appendix, Fig. S3). No significant interaction clusters were revealed. In post hoc analyses for genotype, male mice showed larger volumes for Fkbp5Nex > Fkbp5lox/lox in the left caudoputamen, right piriform area, bilateral dorsal thalamus and left ventral subiculum (uncorrected, Fig. 3). For Fkbp5Nex < Fkbp5lox/lox, we observed a cluster in the bilateral motor cortex extending to the anterior cingulate area (pFWE,cluster = 0.029) and right barrel field (uncorrected). Again, sex-specific effects on brain volume were demonstrated. Female Fkbp5Nex mice showed a cluster in the right anteriormedial visual area for the contrast Fkbp5Nex < Fkbp5lox/lox (trend: pFWE,cluster < 0.072), as well as a nonsignificant cluster in the left dorsal thalamus and left anteriormedial visual area (Fig. 3).
Loss of FKBP51 in GABAergic Neurons Leads to Most Prominent Molecular Changes in the BNST of Females and in the DHC of Males.
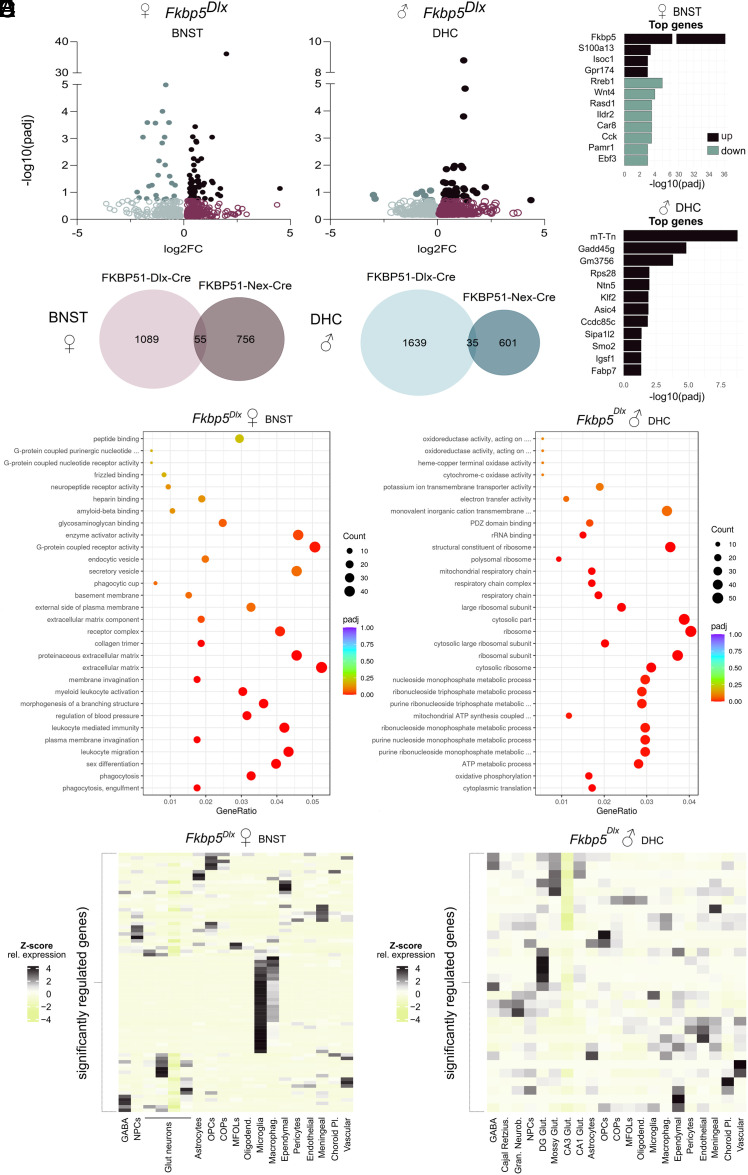
To elaborate further on the molecular pathways that may underly the sex- and FKBP51-manipulated cell type-dependent functional and structural changes that were observed as a result of loss of FKBP51, RNA was extracted from two brain regions: the BNST and the DHC. The BNST is known to be involved in anxiety disorders (44, 45) and as behavioral analyses indicated opposing changes in anxiety-like behavior for Fkbp5Nex and Fkbp5Dlx female mice and the structural analyses revealed deformations around the BNST in female Fkbp5Dlx mice, this region was selected to explore underlying molecular changes. In addition, loss of FKBP51 affected spatial memory performance in an aversive context, exclusively in male mice. Since the DHC is known to be the major brain region involved in spatial memory formation and the MRI analyses indicated large volumetric increases in male Fkbp5Dlx vs. Fkbp5lox/lox mice, it was chosen as the second region of interest. Following the RNA extraction, BNST and DHC samples of all conditions were sequenced. Bioinformatic analyses of the sequencing data revealed a differential expression pattern for each of the eight comparisons. However, only a selection of these differentially expressed genes (DEGs) survived additional statistical correction (Table 1). In line with the structural changes mentioned above and once more emphasizing the sex and cell-type dependency, the most profound significant DEG profiles were found within Fkbp5Dlx (vs. Fkbp5lox/lox) female mice in the BNST area and in Fkbp5Dlx (vs. Fkbp5lox/lox) male mice within the DHC (Fig. 4A). Male and female Fkbp5Dlx mice had respectively a 17-fold larger (52 vs. 3 significant DEGs) and 30-fold larger (91 vs. 3 significant DEGs) differential transcriptional profile than Fkbp5Nex mice of matching sex in the same region. Gene expression profiles also were unique for Fkbp5Dlx and Fkbp5Nex mice as for females in the BNST there was only a 3% overlap of DEGs and for male mice in the DHC a 1.5% overlap of differentially regulated transcripts was found (Fig. 4B). Fkbp5Dlx female mice had a regulatory transcriptional signature in the BNST with 70% upregulated genes and 30% downregulated genes, whereas Fkbp5Dlx male mice had 83% upregulated and only 17% downregulated transcripts. This was reflected in the top 12 most significant DEGs which contained both up- and downregulated genes for female Fkbp5Dlx mice, but for male Fkbp5Dlx mice only consisted of downregulated genes (Fig. 4C).
Table 1.
Differential expression profile for all brain regions, sexes and genetic conditions
| Brain region | Sex | Comparison | No. of ↑ genes corrected (Uncorrected) | No. of ↓ genes corrected (Uncorrected) |
|---|---|---|---|---|
| BNST | Female | Fkbp5Dlx vs. Fkbp5lox/lox | 64 (629) | 27 (515) |
| Fkbp5Nex vs. Fkbp5lox/lox | 2 (404) | 1 (407) | ||
| Male | Fkbp5Dlx vs. Fkbp5lox/lox | 0 (436) | 0 (448) | |
| Fkbp5Nex vs. Fkbp5lox/lox | 0 (159) | 0 (119) | ||
| DHC | Female | Fkbp5Dlx vs. Fkbp5lox/lox | 1 (202) | 0 (316) |
| Fkbp5Nex vs. Fkbp5lox/lox | 2 (591) | 2 (589) | ||
| Male | Fkbp5Dlx vs. Fkbp5lox/lox | 43 (1,024) | 9 (650) | |
| Fkbp5Nex vs. Fkbp5lox/lox | 2 (310) | 1 (326) |
Notes: ↑ = upregulated; ↓ = downregulated; corrected = p adjusted < 0.02; uncorrected = p < 0.05
Fig. 4.
Loss of Fkbp5 leads to cell-specific and sex-dependent molecular profiles that are highly region distinctive. (A) A large differential expression profile could be found within the BNST of female Fkbp5Dlx mice and the DHC of male Fkbp5Dlx older aged mice. (B) These regulated transcriptional profiles were unique for Fkbp5Dlx vs. Fkbp5Nex mice. (C) Within the BNST of Fkbp5Dlx female mice, the top significantly DEGs consisted of up- and downregulated genes, whereas the top DEGs for the DHC of Fkbp5Dlx male mice only included downregulated genes. (D) GO-enrichment analyses revealed enriched biological processes, cellular components and molecular functions in samples of our two separate transcription profiles. For the BNST of Fkbp5Dlx female mice, regulated genes were particularly related to immune functions. Regulated genes of the DHC of Fkbp5Dlx male mice were on the other hand predominantly associated with metabolic processes and ribosomal structural and functional mechanisms. Using a VHC single-cell sequencing dataset (E), we found that significant DEGs in the BNST of Fkbp5Dlx female mice are highly enriched in microglia.
To further explore the underlying pathways related to the transcriptional profiles that were found in the BNST of Fkbp5Dlx female and the DHC of male Fkbp5Dlx mice, a gene ontology (GO) enrichment analysis was performed on all DEGs. In Fig. 4D, a dot bar illustrates the most significantly enriched GO terms for biological processes, cellular components, and molecular functions for each of the transcriptional profiles of the Fkbp5Dlx male and female mice. This GO enrichment analyses revealed that the BNST transcriptional signature for Fkbp5Dlx females is associated with immune-related functions [leukocyte migration, leukocyte-mediated immunity, myeloid leukocyte activation, and mechanisms of phagocytosis, including (plasma) membrane invagination] but also with blood pressure regulation and sex differentiation. Moreover, these functions were reflected in the top DEGs, as a number of these genes are known to modulate immune function (S100a13, Isoc1, Gpr174, Wnt4, Ildr2, Cck; Fig. 4C) (68–73). Pathways that were enriched in the DHC DEG profiles for Fkbp5Dlx males on the other hand were predominantly related to cell metabolic processes and mitochondrial and ribosomal structural and functional mechanism. Significant DEGs in the DHC Fkbp5Dlx male sample are implicated in age-related cognitive impairments (Gadd45g, Smo) (74) or play a role in memory and learning (Asic4) (75) or pre-synaptic and autophagic alterations (Sipa1l2) (76).
Within our genetically manipulated mouse lines, loss of FKBP51 was exclusively present in either gamma-aminobutyric acid (GABA) or glutamatergic neurons of the forebrain. However, lack of FKBP51 in a select population of neurons may also alter properties of other cell types in the same brain region. In order to further identify cell types that might be additionally affected by the selective loss of FKBP51, we examined in which cell types the significant DEGs of our samples are enriched. For this, we made use of an at-hand previously obtained hippocampus single-cell RNA sequencing dataset (77). Interestingly, we found that significant DEGs in the Fkbp5Dlx female BNST are generally also highly expressed in microglia (Fig. 4E). This finding is in line with the associated immune function-related pathways that we observed from the GO enrichment analyses and top significant DEGs. In addition to microglia, groups of significant DEGs of the female Fkbp5Dlx BNST also had a relative high expression in neural progenitor cells, glutamatergic cells, astrocytes, oligodendrocyte precursor cells (OPCs), committed OPCs, mature oligodendrocyte, macrophages, ependymal cells, meningeal and vascular cells. These findings indicate that even though Fkbp5 is only lacking in the GABAergic neurons of the forebrain of female mice it may also affect other cell types of the brain, in particular microglia, and could thereby lead to neuroimmune function-related alterations. For the DHC of Fkbp5Dlx male mice we found that the significant DEGs are also generally expressed in cell types such as dentate gyrus and mossy glutamatergic neurons, ependymal, meningeal, choroid plexus, and vascular cell types (Fig. 4E).
Discussion
Since the discovery of FKBP5 as a key genetic risk factor for psychiatric disease (13), a vast amount of work has been put in trying to unravel the exact mechanisms by which it contributes to stress susceptibility and resilience (18–20, 22–27, 31, 32, 49, 50). Part of this work has already highlighted that FKBP51 differentially implements its functions based on the cell type it is expressed in. Moreover, previous studies have shown that sex and age, two strongly determining risk factors for psychiatric illness, can influence FKBP51 functionality. However, there is still a gap in information on the contribution of FKBP51 in specifically glutamatergic or GABAergic neurons to stress coping behavior and how it interacts with sex and age. With this study we have gained more insight in this by demonstrating that the glutamatergic or GABAergic loss of FKBP51 in the forebrain of older aged animals opposingly affects behavior, brain structure and gene expression profiles in a highly sex-dependent fashion.
In line with results from earlier studies, we found that loss of FKBP51 resulted in changes in emotional regulation and cognitive functioning in a stressful environment. Previous studies demonstrated that systemic FKBP51 inhibition with the selective inhibitor SAFit2(25) and manipulation of FKBP51 in the ovBNST affected anxiety-like behavior (27). Other studies have also emphasized the role of FKBP51 in cognitive functioning. For example, overexpression of Fkbp5 resulted in diminished reversal learning in the Morris Water Maze (30) and led to a reduction in neuronal numbers in the hippocampus (31). Moreover, in humans, increased FKBP51 levels were associated with Alzheimer’s disease progression, a neurological disorder that is well known for its devastating progressive memory decline(49). One notable finding in our study was that loss of FKBP51 affected memory function under stressful conditions, but not in a neutral environment. An extensive amount of literature has already described the versatile relationship between stress and memory and learning (78, 79), and as a stress-responsive gene, it is therefore not surprising that our results accentuate the context-dependent nature of FKBP51 actions. Apart from the effects on behavior, loss of FKBP51-induced changes in brain structure. Deformations were found in GM volumes in limbic structures, areas of the cortex and basal ganglia and in a number of WM structures. Structural brain changes have been demonstrated before in relation to FKBP51. A previous study involving Fkbp5−/− mice exposed volumetric changes in limbic, periaqueductal grey and dorsal raphe nuclei regions as a result of full body loss of FKBP51, but, in line with our findings, also detected changes in WM structures such as the anterior commissure (80). In our study, the thalamus was also affected in both Fkbp5Dlx and Fkbp5Nex mice. Interestingly, an MRI study in humans revealed that individuals with decreased methylation at intron 7 of the FKBP5 gene, associated with increased FKBP51 levels (81), have larger right thalamus volumes (82). Moreover, carriers of the FKBP5 SNP risk allele rs13060780 had reduced thalamic GM volumes when they are growing up in a positive parenting environment (83). The thalamus is an important integrator of sensory inputs and behaviors and it has previously shown to be sensitive to the effects of stress (84, 85). Although the exact mechanisms by which Fkbp5 manipulation affects thalamic brain volume still remain unclear, these results suggest that the thalamus may be involved in the emotional regulatory actions of FKBP51. Two other brain regions that are likely involved in the mechanisms by which Fkbp5 manipulation affects behavior, are the hippocampus and the BNST. Not only did we observe volumetric differences in the bilateral hippocampus and GM regions around the BNST, but a robust transcriptomic profile was also found in both regions, substantiating their importance in Fkbp5-mediated effects.
Remarkably, our study revealed that loss of FKBP51 had opposing effects on behavior when it was restricted to different neuronal cell populations. We found that glutamatergic loss led to anxiogenic behaviors and enhanced the memory of an aversive spatial context, whereas reduced FKBP51 in GABAergic neurons resulted in an anxiolytic phenotype and diminished aversive spatial memory formation. This cell-divergent profile was not only observed on a behavioral level, but was also reflected in changes in brain volume and downstream gene expression levels. In general, the strongest effects were found for Fkbp5Dlx mice on all three dimensions. Since glutamatergic neurons principally exert excitatory functions, while GABAergic projections have inhibiting effects, it is understandable that loss of FKBP51 in either cell population leads to opposing functionality. Supporting this, cell-specific effects of genetic manipulation in relation to stress and behavior have already been reported before. Our group, for example, showed that KO of GR in glutamatergic or GABAergic neurons differentially affected fear- and anxiety-like behavior and hypothamalic-pituitary-adrenal axis reactivity (66). Remarkably, loss of FKBP51 in one specific cell type can indirectly lead to molecular changes in other cell populations. We demonstrated that specific manipulation of Fkbp5 in GABAergic neurons resulted in a transcriptomic profile in the BNST that was particularly enriched in microglia. Interestingly, microglia have a high Fkbp5 mRNA expression on their own (52) and an FKBP51-dependent link between neuroimmune regulation and GABAergic neurons has already been suggested (86). Our data further endorse this communication between GABAergic neurons and microglia, with FKBP51 as a mediating factor. Notably, in our study, we found that GABAergic loss of FKBP51 can lead to anxiolytic behavior and improved cognitive functioning and is associated with increased volumes of the hippocampus. This demonstrates that targeted manipulation of a stress-regulating factor like FKBP51 can have beneficial effects on behavior and brain structure. A recent MRI study, using high-resolution structural imaging, showed that exposure to early life trauma led to volumetric increases in specific subregions of the hippocampus and amygdala and proposed that these subregion-specific increases were associated with beneficial outcomes on behavior (87). It is interesting to speculate on that cell-type or region-specific changes in GR-mediated pathways might be underlying these more protective neurobiological mechanisms following exposure to stress in early life. However, without a doubt, it underlines the importance of more region and cell-type specific approaches when studying stress resilience mechanisms.
Another important observation of this study was the clear presence of a sex-dependent phenotype. Basic phenotyping of global KO of FKBP51 has been done previously in male and female mice (18, 20) and in contrast to our cell-type specific approach, it identified no drastic differences in baseline and stress-induced phenotypes between sexes. However, data on male and female global FKBP51 KO comes from separate studies and have used different type of stressors to test for stress reactivity phenotypes. In our study with conditional KO models, however, loss of FKBP51 in females evidentially induced changes in anxiety-like behavior, whereas for male mice, it led to alterations on the cognitive domain. Our data support demographic studies in humans, which have shown on a large scale that anxiety disorders are twice as common in women than in man (55, 88). The sex-dependent distinction that we found in behavior was supported by the observed structural brain changes. Male Fkbp5Dlx mice had a strongly increased volume of the bilateral hippocampus, a brain region that is majorly implicated in spatial memory and learning. Female Fkbp5Dlx mice, on the other hand, had volumetric differences in the areas around the BNST, which is highly associated with fear and anxiety states. To continue along this line, robust differential expression profiles for males were found in the DHC, though for females, downstream gene expression was most strongly altered in the BNST. Matching with the previously mentioned behavioral changes, some of the top regulated genes in male mice lacking FKBP51 in GABAergic neurons were either directly implicated in memory and learning, or were associated with presynaptic function and autophagic changes. The most significant DEGs in females were however involved in immune function regulation, endorsing the enrichment of the transcriptional signature in microglia. Even though sex-dependent transcriptomic profiles following acute or chronic stress exposure have been demonstrated before, particularly data from high-throughput studies is still limited (59). It is therefore extremely important that increasingly more studies highlight sex-divergent effects on many different levels. One limitation of this study was that cohorts of male and female animals did not come from identical breeding pairs or were not tested at the exact same timepoint. Therefore, the additional, direct sex-specific analyses as carried out for physiological and behavioral, data could not be extended for the RNA sequencing data.
Apart from sex, age is a strongly contributing psychiatric risk factor. Therefore, we tested KO of Fkbp5 under the high-risk environment of an older age. Previous studies have investigated the consequences of loss of FKBP51 in younger male and female mice and did not observe changes in emotional regulation or cognitive functioning under baseline conditions (18, 20). Even though these studies investigated behaviors in full-body KO animals, while in our sample loss of FKBP51 was restricted to the glutamatergic or GABAergic neurons, it is plausible that the lack in effect on behavior can be subscribed to the younger age of the animals. This conception is substantiated by a study from Sabbagh and colleagues that have demonstrated an additive antidepressant effect of KO of Fkbp5 with increasing age (50). This observation may in part be explained by underlying epigenetic changes that lead to accumulated intracellular FKBP51 levels, which impose a higher risk for developing psychiatric symptoms (30, 31, 81).
In summary, we demonstrated that under the high-risk environment of an older age, loss of FKBP51 in GABA or glutamatergic neurons led to unique and strongly sex-dependent outcomes on multiple levels. The outcomes of this study once again corroborate the importance of FKBP51 in emotional regulation and cognitive functioning, even under baseline conditions. As our data highlight that manipulation of FKBP51 leads to highly unique phenotypes dependent on the cell type, this emphasizes the need for cell-specific target treatments. Even more so, it underlines the extreme importance to consider sex when studying stress resilience mechanisms and to ultimately recognize this differential profile in treatment strategies.
Materials and Methods
Animals and Housing Conditions.
The genetic mouse lines Fkbp5Nex and Fkbp5Dlx were bred in-house at the breeding facility of the Max Planck Institute of Psychiatry in Munich, Germany. Male Fkbp5Nex and Fkbp5Dlx mice that were used for RNAScope validation were 9 to 10 mo old at sacrifice. All experimental animals of both mouse lines and sexes were between 10 and 15 mo of age at the onset of the experiments. Mice were group-housed in individually ventilated cages (IVCs; 30 cm × 16 cm × 16 cm), serviced by a central airflow system (Tecniplast, IVC Green Line—GM500) in a stably controlled environment (12h:12h light/dark cycle, temperature of 23 ± 2 °C and humidity of 55%). Water and food (standard research diet by Altromin 1318, Altromin GmbH, Germany) were provided to the animals ad libitum. Two weeks before the start of experimental testing male mice were single-housed and female mice were pair-housed. All experiments and protocols were performed in accordance with the European Communities’ Council Directive 2010/63/EU and were approved by the committee for the Care and Use of Laboratory animals of the Government of Upper Bavaria. All effort was made to minimize any suffering of the animals throughout the experiments.
Generation of Fkbp5Nex and Fkbp5Dlx Mouse Lines.
Conditional KO of Fkbp5 in glutamatergic neurons (Fkbp5Nex) of the forebrain was achieved by crossing Fkbp5lox/lox mice with Nex-Cre mice (65), where Cre is highly expressed in differentiating neurons of the dorsal telencephalon and is active in the adult mouse brain in glutamatergic neurons of the neocortex, amygdala, olfactory bulb and hippocampus, but not in the dentate gyrus (65, 66). Fkbp5Nex offspring therefore selectively lack Fkbp5 expression in the forebrain glutamatergic neurons, starting from embryonic day 11.5 (65). For a conditional KO of Fkbp5 in GABAergic neurons (Fkbp5Dlx), Fkbp5lox/lox mice were crossed with Dlx5/6-Cre mice, in which Cre is expressed in essentially all GABAergic neurons of the forebrain during development (67). Fkbp5lox/lox littermates were used in as a WT control group in all experiments.
Experimental Setup.
Older aged male and female Fkbp5Nex (males: n = 11 vs. n = 11 Fkbp5lox/lox; females: n = 15 vs. n = 7 Fkbp5lox/lox) and Fkbp5Dlx mice (males: n = 15 vs. n = 13 Fkbp5lox/lox; females: n = 15 vs. 13 Fkbp5lox/lox) were tested as separate cohorts (separated per sex and strain), but with the same experimental timeline. In the week prior to testing, animals were weighed and handled twice to familiarize them with the experimenter. During 4 consecutive days, mice underwent a number of behavioral tests in the following sequence: the OF test, EPM test, and NOR and SOR test. Following 2 to 4 rest days, mice were exposed to a 2-d fear-conditioned context retrieval paradigm. Subsequently, 4 to 7 wk succeeding behavioral testing, animals underwent a structural MRI scan. Finally, all mice were weighed and sacrificed 2 wk after the MRI scan.
Behavioral Protocol.
A 6-d behavioral protocol was set up in order to study the effects of loss of FKBP51 in either glutamatergic or GABAergic forebrain neurons on a number of behavioral domains, including tests assessing locomotor activity (OF), anxiety-like behaviors (EPM) and cognitive functioning (NOR and SOR, contextual fear conditioning). All behavior tests were performed in the light phase between 7 AM and 1 PM. During all behavioral tests, animals were recorded with an external camera device and behaviors were later tracked using the advanced video tracking software ANY-maze v.7.15 (Stoelting). In case manual tracking was necessary, an experienced observer was blinded to the group allocation. Please find a more detailed description of each of the behavioral tests in SI Appendix.
MRI.
Structural MRI was performed in a horizontal BRUKER Biospec 94/20 animal scanner (Bruker BioSpin), operating at 9.4 Tesla and using a transmit/receive cryo-coil with two coil elements, as described previously (89). For more details see SI Appendix.
Tissue Sampling.
Animals were sacrificed by decapitation immediately following anesthesia with isofluorane. Baseline trunk blood was collected in 1.5 mL EDTA-coated microcentrifuge tubes (Kabe Labortechnik), centrifuged for 15 min at 8,000 rpm at 4 °C and stored at −20 °C. Furthermore, adrenals and brains were dissected. After collection, adrenals were washed in 9% NaCl, dried and weighed. Brains were snap-frozen in methyl butane on dry-ice and stored at −80 °C.
RNAScope mRNA In Situ Hybridization.
RNAScope mRNA in situ hybridization was performed on male mice of both conditional KO lines and Fkbp5lox/lox controls (3 Fkbp5Nex vs. 3 Fkbp5lox/lox and 4 Fkbp5Dlx vs. 4 Fkbp5lox/lox). For details see SI Appendix.
RNA Sequencing.
RNA extraction.
Frozen brains were mounted in a cryostat microtome and punches of the BNST and DHC were collected in 1.5 mL DNA LoBind Safe-lock Eppendorf tubes, using a punching tool with a diameter of 1 mm. Tissue of six biological replicates per condition was immediately saved on dry ice and later stored at −80 °C. RNA extraction was then achieved by making use of the miRNeasy Mini Kit (cat. no. 1038703, QIAGEN) according to the manufacturer’s protocol.
RNA sequencing and differential expression analysis.
RNA quality control, library preparation, transcriptome sequencing, and bioinformatic analyses were performed on-site by the company Novogene UK (Novogene Europe) according to their standardized protocols. For the differential expression analyses, eight comparisons were setup (a KO vs. WT comparison was performed for each of the genetic mouse lines, the different sexes and two separate brain regions). Genes with adjusted P value (q) < 0.02 and log2 fold change were referred to as significantly differentially regulated genes.
Enrichment analyses.
GO enrichment analysis of DEGs was implemented by the clusterProfiler R package. GO terms with a corrected P value < 0.05 were considered significantly enriched by DEGs. At last, a single-cell RNA sequencing dataset of the mouse hippocampus was used to check in which cell types significant DEGs are enriched (77).
Statistical Analyses.
Statistical analyses for physiological, behavioral and RNAScope data were performed in R studio (R.4.2.0). Statistical assumptions were then checked by using a Shapiro-Wilk test for Normality and a Levene’s test to check for equality of variances. If data violated these assumptions, nonparametric statistical tests were used or a boxcox transformation was applied to normalize the data. Subsequently, two-group comparisons were performed with an independent t test or a nonparametric Wilcoxon test. As cohorts of the female Fkbp5Nex and male Fkbp5Nex and Fkbp5Dlx mice had varying age at baseline in control and conditional KO groups, for these cohorts a one-way analysis of covariance (ANCOVA) with age in weeks at baseline as a covariate was conducted. Before analyses, additional assumption checks for the one-way ANCOVA were done, including checks for linearity between covariate and dependent variable, homogeneity of the regression slopes and a normality check for the residuals. As cohorts of male and female animals were tested at different timepoints and these cohorts of animals resulted from different breeding pairs, the initial analysis did not include “sex” as a factor. However, in order to further emphasize interesting sex effects, an additional two-way ANCOVA with sex and genotype as independent factors was applied to the data of the Fkbp5Nex and Fkbp5Dlx cohorts. Results from these analyses are given in SI Appendix, Table S1. Values that were greater or smaller than two times the SD from the mean (M) were considered outliers and were excluded from analyses. Graphs were created with GraphPad Prism 9 and all remaining data illustrations were composed in R studio. Part of the figures was composed with the help of Biorender.com. P values of less than 0.05 were considered statistically significant and a statistical trend was recognized for P values of 0.1 ≥ P ≥ 0.05.
For MRI data a two-way ANOVA (sex × genotype and interaction) with Tukey post hoc testing was used on the total brain volume (TBV) and on the tissue compartments to evaluate differences in brain tissue composition and total brain size. Smoothed Jacobian deformation fields were compared in SPM12 in an independent two-factorial model (genotype × sex) for Fkbp5Dlx and Fkbp5Nex mice vs. their Fkbp5lox/lox controls, respectively. Analyses included TBV as a covariate. As TBV differed for sexes, we introduced the TBV covariate split according to sex (i.e., independent group mean values for male and female values). If not stated otherwise, reported results survive an FWE correction at the cluster level (pFWE,cluster < 0.05), with a cluster collection threshold of P < 0.005 uncorrected.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Daniela Harbich and Bianca Schmid for their excellent technical assistance and support. We also thank Stefani Unkmeir, Sabrina Bauer, and the scientific core unit Genetically Engineered Mouse Models for genotyping assistance. This work was supported by SCHM2360/31 grant (to M.V.S.) from the German Research Foundation (DFG), the “Kids2Health” grant of the Federal Ministry of Education and Research (01GL1743C; to M.V.S.).
Author contributions
L.v.D. and M.V.S. designed research; L.v.D., T.S., S.M., H.Y., J.B., L.S., C.E., V.K., S.N., L.M.B., M.S., J.P.L., and M.C. performed research; J.M.D. contributed new reagents/analytic tools; L.v.D. and M.C. analyzed data; and L.v.D. and M.V.S. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
RNAseq data have been deposited in Gene Expression Omnibus under accession number GSE232460 (90). All other data are included in the manuscript and/or SI Appendix.
Supporting Information
References
- 1.Pariante C. M., Miller A. H., Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biol. Psychiatry 49, 391–404 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Brown E. S., Varghese F. P., McEwen B. S., Association of depression with medical illness: Does cortisol play a role? Biol. Psychiatry 55, 1–9 (2004). [DOI] [PubMed] [Google Scholar]
- 3.de Kloet E. R., Joëls M., Holsboer F., Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 6, 463–475 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Vreeburg S. A., et al. , Salivary cortisol levels in persons with and without different anxiety disorders. Psychosomatic Med. 72, 340–347 (2010). [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO), Depression and Other Common Mental Disorders: Global Health Estimates (WHO, 2017). [Google Scholar]
- 6.The Lancet Global. Health, Mental health matters. Lancet Global Health 8, e1352 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santomauro D. F., et al. , Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 398, 1700–1712 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asmundson G. J. G., et al. , Do pre-existing anxiety-related and mood disorders differentially impact COVID-19 stress responses and coping? J. Anxiety Disorders 74, 102271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendler K. S., Karkowski L. M., Prescott C. A., Causal relationship between stressful life events and the onset of major depression. AJP 156, 837–841 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Caspi A., Moffitt T. E., Gene–environment interactions in psychiatry: Joining forces with neuroscience. Nat. Rev. Neurosci. 7, 583–590 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Belsky J., et al. , Vulnerability genes or plasticity genes? Mol. Psychiatry 14, 746–754 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zannas A. S., Binder E. B., Gene-environment interactions at the FKBP5 locus: Sensitive periods, mechanisms and pleiotropism: FKBP5 and gene-environment interactions. Genes, Brain Behav. 13, 25–37 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Binder E. B., et al. , Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 36, 1319–1325 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Ellsworth K. A., et al. , FKBP5 genetic variation: Association with selective serotonin reuptake inhibitor treatment outcomes in major depressive disorder. Pharmacogenet. Genomics 23, 156–166 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Psychiatric GWAS Consortium Coordinating Committee, Genomewide Association Studies: History, Rationale, and Prospects for Psychiatric Disorders. AJP 166, 540–556 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binder E. B., The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 34, S186–195 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Wochnik G. M., et al. , FK506-binding Proteins 51 and 52 Differentially Regulate Dynein Interaction and Nuclear Translocation of the Glucocorticoid Receptor in Mammalian Cells. J. Biol. Chem. 280, 4609–4616 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Touma C., et al. , FK506 binding protein 5 shapes stress responsiveness: Modulation of neuroendocrine reactivity and coping behavior. Biol. Psychiatry 70, 928–936 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Hartmann J., et al. , The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology 62, 332–339 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Hoeijmakers L., et al. , Depletion of FKBP51 in female mice shapes HPA axis activity. PLoS ONE 9, e95796 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fries G., Gassen N., Rein T., The FKBP51 Glucocorticoid receptor co-chaperone: Regulation, function, and implications in health and disease. IJMS 18, 2614 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie P., et al. , Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacol 35, 1684–1692 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klengel T., et al. , Allele-specific FKBP5 DNA demethylation mediates gene–childhood trauma interactions. Nat. Neurosci. 16, 33–41 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Leary J. C., et al. , A new anti-depressive strategy for the elderly: Ablation of FKBP5/FKBP51. PLoS ONE 6, e24840 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmann J., et al. , Pharmacological inhibition of the psychiatric risk factor FKBP51 has anxiolytic properties. J. Neurosci. 35, 9007–9016 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Häusl A. S., et al. , The co-chaperone Fkbp5 shapes the acute stress response in the paraventricular nucleus of the hypothalamus of male mice. Mol. Psychiatry 26, 3060–3076 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelhardt C., et al. , FKBP51 in the oval bed nucleus of the stria terminalis regulates anxiety-like behavior. eNeuro 8, ENEURO.0425-21.2021 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brix L. M., et al. , Contribution of the co-chaperone FKBP51 in the ventromedial hypothalamus to metabolic homeostasis in male and female mice. Mol. Metab. 65, 101579 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Häusl A. S., et al. , Mediobasal hypothalamic FKBP51 acts as a molecular switch linking autophagy to whole-body metabolism. Sci. Adv. 8, eabi4797 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blair L. J., et al. , The disease-associated chaperone FKBP51 impairs cognitive function by accelerating AMPA receptor recycling. eNeuro 6, ENEURO.0242-18.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Criado-Marrero M., et al. , FKBP5 and early life stress affect the hippocampus by an age-dependent mechanism. Brain Behav. Immun. Health 9, 100143 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharf S. H., Liebl C., Binder E. B., Schmidt M. V., Müller M. B., Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS One 6, e16883 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe Y., Gould E., McEwen B. S., Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 588, 341–345 (1992). [DOI] [PubMed] [Google Scholar]
- 34.Sapolsky R. M., Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatry 57, 925 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Campbell S., Macqueen G., The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 29, 417–426 (2004). [PMC free article] [PubMed] [Google Scholar]
- 36.Malykhin N. V., Carter R., Seres P., Coupland N. J., Structural changes in the hippocampus in major depressive disorder: Contributions of disease and treatment. jpn 35, 337–343 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stockmeier C. A., et al. , Cellular changes in the postmortem hippocampus in major depression. Biol. Psychiatr. 56, 640–650 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKinnon M. C., Yucel K., Nazarov A., MacQueen G. M., A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J. Psychiatr. Neurosci. 34, 41–54 (2009). [PMC free article] [PubMed] [Google Scholar]
- 39.Cobb J. A., et al. , Hippocampal volume and total cell numbers in major depressive disorder. J. Psychiatr. Res. 47, 299–306 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeisel A., et al. , Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Erö C., Gewaltig M.-O., Keller D., Markram H., A cell atlas for the mouse brain. Front. Neuroinform. 12, 84 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henke P. G., Hippocampal pathway to the amygdala and stress ulcer development. Brain Res. Bull. 25, 691–695 (1990). [DOI] [PubMed] [Google Scholar]
- 43.Jimenez J. C., et al. , Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 97, 670–683.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis M., Walker D. L., Miles L., Grillon C., Phasic vs. sustained fear in rats and humans: Role of the extended amygdala in fear vs. anxiety. Neuropsychopharmacol 35, 105–135 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lebow M. A., Chen A., Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 21, 450–463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glaesmer H., Riedel-Heller S., Braehler E., Spangenberg L., Luppa M., Age- and gender-specific prevalence and risk factors for depressive symptoms in the elderly: A population-based study. Int. Psychogeriatr. 23, 1294–1300 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Lenze E. J., et al. , The course of functional decline in older people with persistently elevated depressive symptoms: Longitudinal findings from the Cardiovascular Health Study. J. Am. Geriatr. Soc. 53, 569–575 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Naismith S. L., Norrie L. M., Mowszowski L., Hickie I. B., The neurobiology of depression in later-life: Clinical, neuropsychological, neuroimaging and pathophysiological features. Prog. Neurobiol. 98, 99–143 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Blair L. J., et al. , Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J. Clin. Invest. 123, 4158–4169 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabbagh J. J., et al. , Age-associated epigenetic upregulation of the FKBP5 gene selectively impairs stress resiliency. PLoS One 9, e107241 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shannon Weickert C., Webster M. J., Boerrigter D., Sinclair D., FKBP5 messenger RNA increases after adolescence in human dorsolateral prefrontal cortex. Biol. Psychiatr. 80, e29–e31 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Matosin N., et al. , Associations of psychiatric disease and ageing with FKBP5 expression converge on superficial layer neurons of the neocortex. Acta Neuropathol. 145, 439–459 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heo M., Murphy C. F., Fontaine K. R., Bruce M. L., Alexopoulos G. S., Population projection of US adults with lifetime experience of depressive disorder by age and sex from year 2005 to 2050. Int. J. Geriat. Psychiatry 23, 1266–1270 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrari A. J., et al. , Global variation in the prevalence and incidence of major depressive disorder: A systematic review of the epidemiological literature. Psychol. Med. 43, 471–481 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Kessler R. C., Lifetime and 12-Month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the national comorbidity survey. Arch. Gen. Psychiatry 51, 8 (1994). [DOI] [PubMed] [Google Scholar]
- 56.Gutiérrez-Lobos K., Scherer M., Anderer P., Katschnig H., The influence of age on the female/male ratio of treated incidence rates in depression. BMC Psychiatry 2, 3 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Doeselaar L., et al. , Chronic social defeat stress in female mice leads to sex-specific behavioral and neuroendocrine effects. Stress 24, 168–180 (2021). [DOI] [PubMed] [Google Scholar]
- 58.Rincón-Cortés M., Herman J. P., Lupien S., Maguire J., Shansky R. M., Stress: Influence of sex, reproductive status and gender. Neurobiol. Stress 10, 100155 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brivio E., Lopez J. P., Chen A., Sex differences: Transcriptional signatures of stress exposure in male and female brains. Genes Brain Behav. 19 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Hodes G. E., Epperson C. N., Sex differences in vulnerability and resilience to stress across the life span. Biol. Psychiatry 86, 421–432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bangasser D. A., Valentino R. J., Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Front. Neuroendocrinol. 35, 303–319 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dalla C., et al. , Chronic mild stress impact: Are females more vulnerable? Neuroscience 135, 703–714 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Merz C. J., Wolf O. T., Sex differences in stress effects on emotional learning: Sex Differences, Stress, and Emotional Learning. J. Neurosci. Res. 95, 93–105 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Bourke C. H., Harrell C. S., Neigh G. N., Stress-induced sex differences: Adaptations mediated by the glucocorticoid receptor. Hormones Behav. 62, 210–218 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goebbels S., et al. , Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis 44, 611–621 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Hartmann J., et al. , Forebrain glutamatergic, but not GABAergic, neurons mediate anxiogenic effects of the glucocorticoid receptor. Mol. Psychiatry 22, 466–475 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Monory K., et al. , The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51, 455–466 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sacerdote P., Ruff M. R., Pert C. B., Cholecystokinin and the immune system: Receptor-mediated chemotaxis of human and rat monocytes. Peptides 9, 29–34 (1988). [DOI] [PubMed] [Google Scholar]
- 69.Jia X., et al. , CCK8 negatively regulates the TLR9-induced activation of human peripheral blood pDCs by targeting TRAF6 signaling. Eur. J. Immunol. 44, 489–499 (2014). [DOI] [PubMed] [Google Scholar]
- 70.Hecht I., et al. , ILDR2 is a novel B7-like protein that negatively regulates T cell responses. J. Immunol. 200, 2025–2037 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Podojil J. R., et al. , ILDR2-Fc is a novel regulator of immune homeostasis and inducer of antigen-specific immune tolerance. J.I. 200, 2013–2024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei T., et al. , Wnt4 signaling is associated with the decrease of proliferation and increase of apoptosis during age-related thymic involution. Mol. Med. Rep. 12, 7568–7576 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Xia C., Braunstein Z., Toomey A. C., Zhong J., Rao X., S100 proteins as an important regulator of macrophage inflammation. Front. Immunol. 8, 1908 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brito D. V. C., Kupke J., Gulmez Karaca K., Zeuch B., Oliveira A. M. M., Mimicking age-associated Gadd45γ dysregulation results in memory impairments in young adult mice. J. Neurosci. 40, 1197–1210 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y.-J., et al. , Follistatin mediates learning and synaptic plasticity via regulation of Asic4 expression in the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 118, e2109040118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andres-Alonso M., Kreutz M. R., Karpova A., Autophagy and the endolysosomal system in presynaptic function. Cell Mol. Life Sci. 78, 2621–2639 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lopez J. P., et al. , Ketamine exerts its sustained antidepressant effects via cell-type-specific regulation of Kcnq2. Neuron 110, 2283–2298.e9 (2022). [DOI] [PubMed] [Google Scholar]
- 78.Abrari K., Rashidy-Pour A., Semnanian S., Fathollahi Y., Jadid M., Post-training administration of corticosterone enhances consolidation of contextual fear memory and hippocampal long-term potentiation in rats. Neurobiol. Learn Mem. 91, 260–265 (2009). [DOI] [PubMed] [Google Scholar]
- 79.Joëls M., Pu Z., Wiegert O., Oitzl M. S., Krugers H. J., Learning under stress: How does it work? Trends Cogn. Sci. 10, 152–158 (2006). [DOI] [PubMed] [Google Scholar]
- 80.Engelhardt C., Boulat B., Czisch M., Schmidt M. V., Lack of FKBP51 shapes brain structure and connectivity in male mice. J. Magn. Reson. Imaging 53, 1358–1365 (2021). [DOI] [PubMed] [Google Scholar]
- 81.Klengel T., Binder E. B., FKBP5 allele-specific epigenetic modification in gene by environment interaction. Neuropsychopharmacol 40, 244–246 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Womersley J. S., et al. , FKBP5 intron 7 methylation is associated with higher anxiety proneness and smaller right thalamus volume in adolescents. Brain Struct. Funct. 227, 2809–2820 (2022), 10.1007/s00429-022-02577-9. [DOI] [PubMed] [Google Scholar]
- 83.Matsudaira I., et al. , rs1360780 of the FKBP5 gene modulates the association between maternal acceptance and regional gray matter volume in the thalamus in children and adolescents. PLoS One 14, e0221768 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoshii T., et al. , Brain atrophy in the visual cortex and thalamus induced by severe stress in animal model. Sci. Rep. 7, 12731 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kooiker C. L., Birnie M. T., Baram T. Z., The paraventricular thalamus: A potential sensor and integrator of emotionally salient early-life experiences. Front. Behav. Neurosci. 15, 673162 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gan Y.-L., et al. , FKBP51 mediates resilience to inflammation-induced anxiety through regulation of glutamic acid decarboxylase 65 expression in mouse hippocampus. J. Neuroinflammation 19, 152 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Picci G., et al. , Amygdala and hippocampal subregions mediate outcomes following trauma during typical development: Evidence from high-resolution structural MRI. Neurobiol. Stress 18, 100456 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Otten D., et al. , Similarities and differences of mental health in women and men: A systematic review of findings in three large german cohorts. Front. Public Health 9, 553071 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruat J., et al. , Structural correlates of trauma-induced hyperarousal in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 111, 110404 (2021). [DOI] [PubMed] [Google Scholar]
- 90.Doeselaar L. v., Schmidt M. V., RNAseq data of hippocampus and BNST in mice lacking FKBP5 in glutamatergic or GABAergic neurons. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE232460. Deposited 12 May 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
RNAseq data have been deposited in Gene Expression Omnibus under accession number GSE232460 (90). All other data are included in the manuscript and/or SI Appendix.