Figure 6. Gβγ plays a key role in reinforcing allosteric pathways and signal transmission.
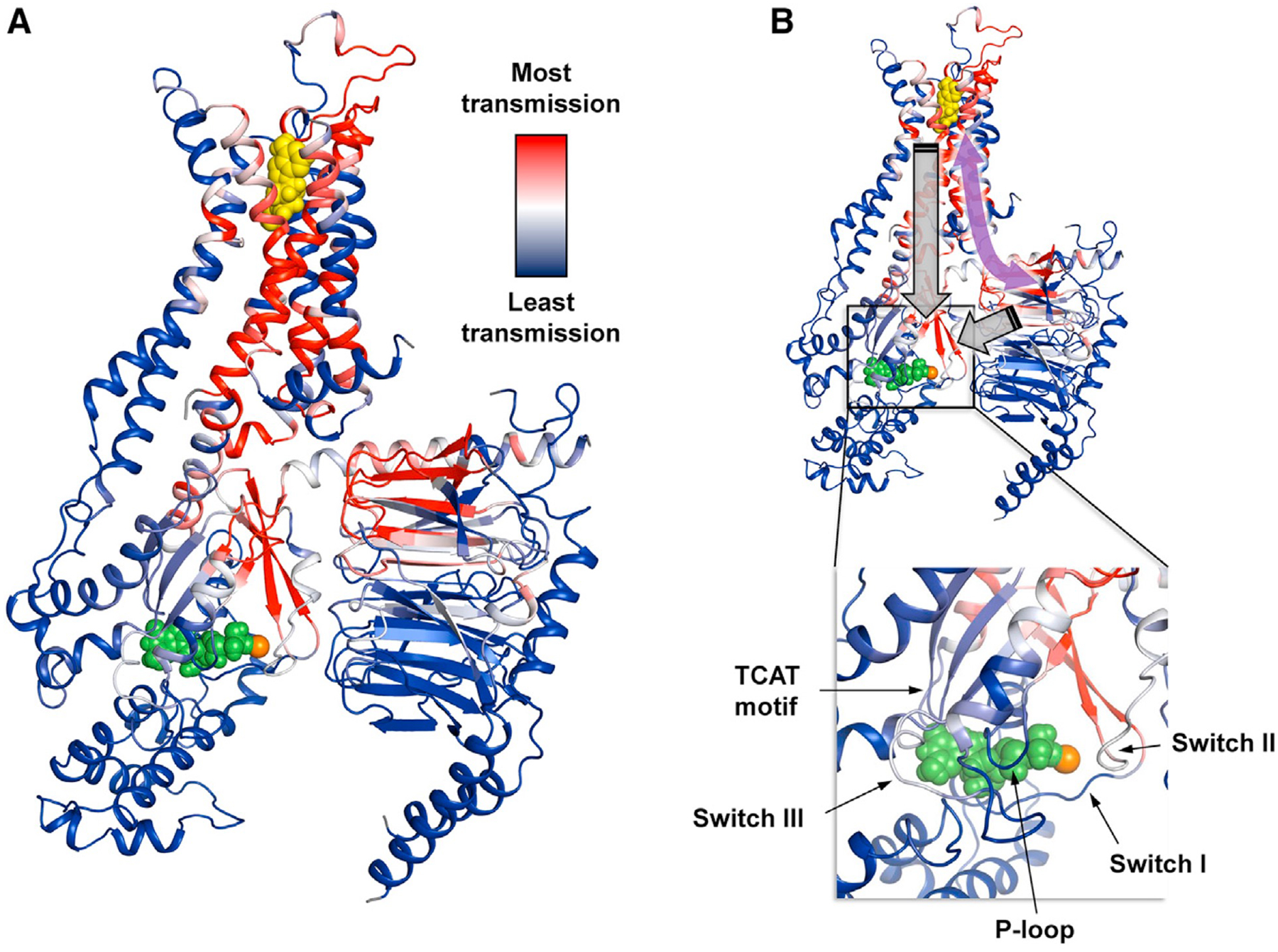
(A) The allosteric network within the ternary complex is revealed through rigidity theory analysis. Here, allosteric transmission is measured by regiospecific changes in degrees of freedom (red/blue color gradient bar) experienced upon rigidification of the agonist NECA (yellow spheres). An allosteric pathway can be defined between the orthosteric pocket and Gsαβγ that, in turn, connects with the nucleotide-binding region. Green spheres designate GDP, and the orange sphere represents Mg2+. (B) The symmetric property of allosteric transmission means that Gβγ, despite not being in direct contact with the receptor, may impart allosteric effects on remote regions in the pathway such as the orthosteric binding site (curved purple arrow). Nucleotide exchange involves structural rearrangement of Gα facilitated by movements of conserved motifs (annotated inset). This likely requires a concerted interplay between receptor and both the Gα and Gβγ subunits acting on the nucleotide-binding pocket (gray block arrows).
