Abstract
The goal of our laboratory is to study the mechanisms that promote nicotine use, particularly in vulnerable populations. To more closely mimic human use patterns, the present study employed nicotine vapor methods involving passive exposure for 14 days in adolescent and adult female and male rats. Age and sex differences in approach behavior (nosepokes) were assessed in a port that delivered nicotine plumes on Day 1 and 14 of our exposure regimen. Controls received ambient air in exposure chambers. After the final session, rats received a nicotinic receptor antagonist to precipitate withdrawal. Then, physical signs, anxiety-like behavior, and plasma levels of cotinine (a nicotine metabolite) were assessed. Over time, females displayed a larger increase in approach behavior to the nicotine port than males, an effect that was larger in adolescents. Nosepoke responses in adolescent females were correlated with anxiety-like behavior, but not physical signs of withdrawal. Adolescents gained more weight than adults regardless of treatment, and the weight gain was larger in male adolescents. Female adolescents also displayed the highest levels of cotinine than all other groups. These findings suggest that nicotine vapor produces greater motivational effects in adolescent females as compared to their adult and male counterparts.
Keywords: reward, motivation, e-cigarette, rat, plumes
In recent years, there has been a significant rise in recreational e-cigarette use along with an increase in dual use with traditional cigarettes (King et al., 2015; Pepper & Brewer, 2014). Unfortunately, the safety of e-cigarettes has been questionable and there are emerging concerns regarding gateway effects in adolescents and females. Indeed, adolescents who use e-cigarettes are up to four times more likely to smoke combustible cigarettes regularly as adults, a relationship that is stronger in women versus men (Chen et al., 2017). Adult women use two-fold higher concentrations of nicotine in e-cigarettes and display greater symptoms of nicotine dependence than men (Pang et al., 2020). Women who smoke combustible cigarettes are also more likely to have tried e-cigarettes than men (Zhu et al., 2013). This is concerning because women are more susceptible than men to the long-term consequences of smoking, which include reproductive problems, pulmonary disease, and cancer (Kong & Krishnan-Sarin, 2017). These epidemiological trends require the application of preclinical studies to provide a deeper understanding of the factors that promote age and sex differences in nicotine use in humans.
Previous work has studied withdrawal in rodents by implanting a subcutaneous pump that delivers nicotine and then removing the pump or administering a nicotinic acetylcholine receptor antagonist (such as mecamylamine) to precipitate withdrawal. The pump model is limited with regard to surgical interventions, the relatively larger size of the pump in adolescent versus adult and female versus male rats, and continuous delivery of nicotine that does not mimic the repeated abstinence periods in human use patterns. Recent advances in the field have employed inhalation methods to induce nicotine dependence in rats (George et al., 2010; Gilpin et al., 2014; Javadi-Paydar et al., 2019; Kallupi et al., 2019; Montanari et al., 2020) and mice (Lefever et al., 2017; Ponzoni et al., 2015). Prior work using nicotine vapor methods in rats has utilized male adults, leaving remaining questions regarding age and sex differences produced by withdrawal from nicotine vapor. To address this issue, the present study assessed nosepoke responses in a port that delivered nicotine plumes in a passive vapor inhalation system. Following nicotine vapor exposure, age and sex differences in physical signs and anxiety-like behavior were compared following precipitated withdrawal. Group differences in weight gain and plasma levels of cotinine (a nicotine metabolite) were also assessed on the final day of the vapor regimen.
Method
Subjects
Adult female (n = 6 nicotine and n = 6 control), adult male (n = 6 nicotine and n = 6 control), adolescent female (n = 6 nicotine and n = 6 control), and adolescent male (n = 6 nicotine and n = 6 control) rats were used. The rats were bred in-house from an outbred stock of Wistar rats (Envigo, Inc., Indianapolis, IN). On postnatal day (PND) 21, the rat pups were weaned and pair-housed with a same-sex litter mate for the remainder of the study. The rats were housed in a humidity- and temperature-controlled (22°C) vivarium on a reverse 12-hr light/dark cycle (lights off between 8:00 a.m. and 8:00 p.m.) with access to water and food ad libitum. All procedures were approved by the UTEP Institutional Animal Care and Use Committee in compliance with the Guide for the Care and Use of Animals (National Research Council, 2010).
Experimental Procedures
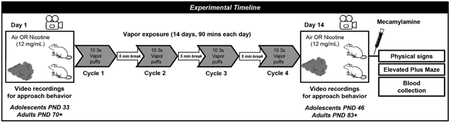
The inset depicts our vapor exposure regimen and test procedures. Before the start of the study, the rats were handled for at least 5 min each day in the vivarium for 5 days. The exposure procedures utilized a Benchtop Passive E-Vape Inhalation System from La Jolla Alcohol Research Inc. (La Jolla, CA). Separate pairs of rats were exposed to either nicotine (12 mg/mL) or ambient air (controls) for 90 min each day for 14 consecutive days. The pairs of rats were derived from the same group condition (i.e., a female adolescent with another female adolescent) and they remained with the same partner throughout the exposure procedure. Our decision to use ambient air for the control condition was based on previous studies from our laboratory showing that PG/VG elicits behavioral effects, such as changes in riskiness, that could impact our assessments of approach behavior (Giner et al., 2022). Other laboratories have also used ambient air as a control condition in nicotine vapor studies in rats (Gilpin et al., 2014).
Each day, the rats were exposed to 90-min sessions consisting of four cycles, with 5-min intercycle intervals. For each cycle, nicotine e-liquid was heated to 400°F for a 3-s puff delivery, occurring every 2 min and 10 times per cycle, for a total of 40 puff deliveries per day. Each cycle duration was 18 min and 30 s. We used flavorless e-liquids containing nicotine in its freebase form in 50/50 vegetable glycerin/propylene glycol (PG/VG) vehicle.
The rationale for our procedures was based largely on prior studies in our laboratory and others using nicotine vapor in rats (Flores et al., 2022; George et al., 2010; Gilpin et al., 2014; Javadi-Paydar et al., 2019; Kallupi et al., 2019; Montanari et al., 2020; Smith et al., 2020). We recognize the need to examine age and sex differences using longer sessions across an extended period than was used here in order to mimic extended daily use patterns in humans. Our e-liquid concentration of nicotine (12 mg/mL) is a moderate concentration that falls within a range of e-liquid concentrations preferred by human e-cigarette users (Etter, 2016; Flouris et al., 2013). Our flavorless e-liquids are purchased in bulk from a commercial vendor (Vapor Vapes Inc, Sand City, CA) that is a popular choice among e-cigarette users. While these liquids provide a better model of human e-cigarette use, it is acknowledged that they may vary in dose and contain contaminants.
The 14-day exposure procedure was done in adolescent rats between PND 33-46 and adult rats between PND 70-88. Two cage mates were exposed in the same chamber throughout the exposure procedure. The exposure system consisted of four sealed chambers (interior dimension of 14.5“ L x 10.5” W x 9.0” H), each with two valve ports. One valve port was connected to a small vacuum that controlled the airflow in the chamber at 0.6 L per minute. The vacuum outlet was connected to a Whatman HEPA filter (Millipore Sigma, Darmstadt, Germany) and onto a house exhaust that safely removed the nicotine from the chambers and outside the testing room. The other valve port was connected via PVC tubing to a modified 4.9-volt TFV4 minitank (Smok Inc, Shenzhen, China) where the nicotine was heated. The minitanks were also linked to a control box that allowed for controlled heating of nicotine e-cigarette liquid (e-liquid). To minimize contamination, the chambers were carefully cleaned after every exposure session, and separate PVC tubing and minitanks were used for air controls and nicotine vapor groups.
Behavioral Measures
During the exposure regimen, nosepokes in the vapor plume delivery port were recorded on Days 1 and 14. The frequency of port contacts (nosepokes) served as an index of approach behavior that was directed at the port where the nicotine plumes were delivered into the chamber. Nosepoke responses were manually scored by an observer that was blind to the rats’ treatment condition. The videos were scored two separate times manually to capture nosepoke responses for each individual rat of the pairings. In our figures, individual data points are color matched to allow for comparisons in each pair of rats. We did not observe any interference of nosepoking behavior when assessing rat pairs. More animals are needed to confirm this assertion given that there were only three pairs of rats per group in the present dataset.
At the end of the final vapor exposure session on Day 14, the rats were placed into a clear Plexiglas® cage and moved to a dedicated test room that was well lit. Following a 10-min acclimation period, the rats received a subcutaneous injection of mecamylamine (3.0 mg/kg) to precipitate nicotine withdrawal. This dose of mecamylamine elicits physical signs of withdrawal in nicotine-dependent female and male Wistar rats (Torres et al., 2015). Ten minutes later, the physical signs of withdrawal were assessed for an additional 10 min. The observed signs included blinks, writhes, body shakes, teeth chatters, gasps, grooming, licks, and ptosis. Multiple successive counts of any sign required a distinct pause between episodes. Ptosis was counted once per minute.
Following our assessments of physical signs, anxiety-like behavior was assessed using elevated plus maze (EPM) procedures. The rats were transported to another dimly lit room and acclimated for 5 min. The EPM apparatus consisted of four arms (two closed and two open) elevated to a height of 50 cm above the ground. The apparatus was illuminated by a red light suspended from the ceiling. At the beginning of the test, the rats were placed in the center of the EPM facing the open arm. Time spent in the center area and the open versus closed arms was recorded for 5 min. Anxiety-like behavior was operationally defined as an increase in time spent in the closed arms relative to controls. All behavioral measures were assessed by an observer that was unaware of the rats’ treatment condition.
Immediately after behavioral testing, the rats were sacrificed, and blood was collected. Nicotine metabolism was assessed indirectly by comparing cotinine (a nicotine metabolite) levels across experimental conditions. The blood was centrifuged for 15 min at 5000 x g at 4°C. Serum was extracted and stored in 100 μL aliquots at −80° C. The plasma cotinine levels were analyzed using commercially available 96-well plate ELISA kits (OraSure Technologies, Inc., Bethlehem, PA). Standard curves were used to estimate plasma cotinine levels using a Spectra Maxplus spectrophotometer (Molecular Devices Inc, Sunnyvale, CA).
Statistics
The dependent variables included nosepoke responses, weight gain, cotinine levels, time spent in the closed arms of the EPM, and physical signs of withdrawal. The nosepoke data and changes in weight were expressed as a percent change from Day 1 to assess time-dependent effects across treatment groups. Multivariate analysis of variance (ANOVA) was used with sex (female vs. male), age (adolescent vs. adult), and treatment (control vs. nicotine) as between-subject factors. For the nosepoke data, time was included in the ANOVA as a within-subject factor (Days 1 versus 14). Where appropriate, significant interaction effects were further analyzed using post-hoc comparisons (Fisher’s LSD test, p ≤ .05). A Bonferroni correction factor was employed to reduce error inflation with multiple comparisons. The relationship between approach behavior and cotinine levels was assessed using a Pearson correlation coefficient analysis. Data were analyzed using IBM SPSS Statistics for Windows, version 27 (IBM Corp., Armonk, NY).
Results
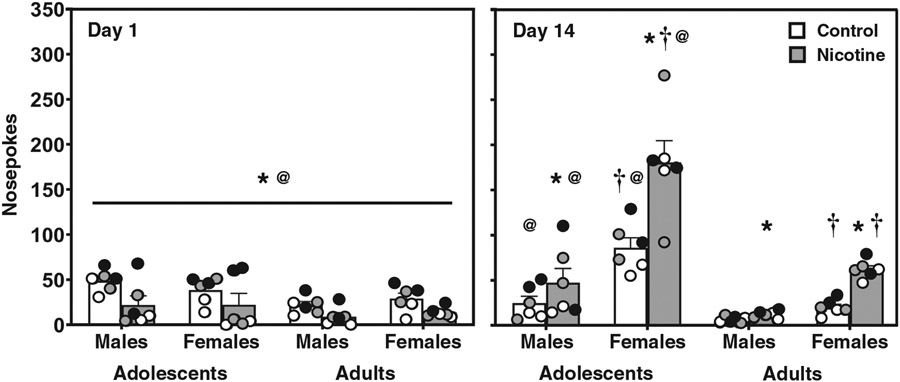
Figure 1 displays nosepokes in control (white bars) and nicotine vapor (grey bars) groups on Days 1 and 14 of the exposure regimen. Overall, the data show that female rats displayed an increase in approach behavior toward a port that delivered nicotine, and this effect was larger in adolescents. The analysis of Day 1 revealed that all rats that received nicotine vapor displayed fewer nosepoke responses than controls (main effect of treatment: F(1,40) = 13.08; *p = .001). Also, adolescent rats displayed more nosepoke responses than adults (main effect of age: F(1,40) = 8.74; @p = .005). The analysis of Day 14 revealed significant interactions between sex and treatment (F(1,40) = 12.61; p = .001), sex and age (F(1,40) = 17.80; p = .001), and treatment and age (F(1,40) = 5.61; p = .02). Post-hoc analyses of these interaction effects revealed that female adolescent controls displayed more nosepoke responses than adult female controls (@p ≤ .05) and adolescent male controls (†p ≤ .05). Female adolescent rats that were exposed to nicotine displayed more nosepoke responses than adolescent female controls (*p ≤ .05) and their adult female (@p ≤ .05) and adolescent male (†p ≤ .05) counterparts. Female adult controls displayed more nosepoke responses than adult male controls (†p ≤ .05). Female adults that were exposed to nicotine displayed more nosepoke responses than adult female controls (*p ≤ .05) and their adult male counterparts (†p ≤ .05). Male adolescent controls displayed more nosepoke responses than adult male controls (@p ≤ .05). Male adolescents that were exposed to nicotine displayed more nosepoke responses than adolescent male controls (*p ≤ .05) and their adult male counterparts (@p ≤ .05). Male adult rats that were exposed to nicotine displayed more nosepoke responses than adult male controls (*p ≤ .05).
Figure 1. Adolescent Females Display More Approach Behavior to a Port That Delivered Nicotine Vapor.
Note. The data reflect mean (± SEM) nosepoke responses in a port that delivered nicotine vapor or ambient air (control) in female and male adolescent and adult rats on Day 1 and Day 14 of the exposure regimen. Individual data points are color matched to allow for comparisons in each pair of rats. The asterisks (*) denote a significant difference from controls, the daggers (†) denote a difference from males, and the at sign (@) denotes a difference from adults (p ≤ .05).
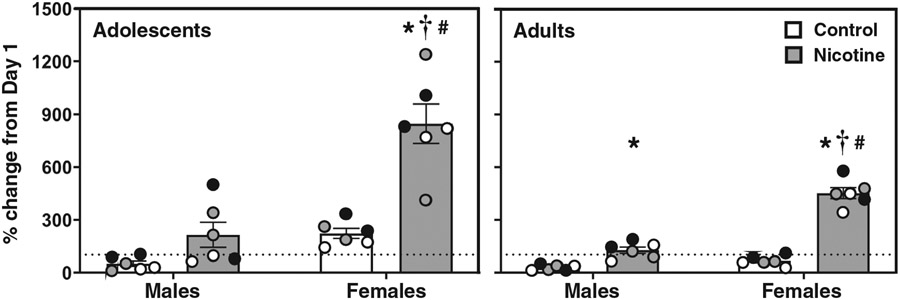
Figure 2 displays nosepoke responses in control (white bars) and nicotine vapor (grey bars) groups on Day 14 expressed as percent change from Day 1. Overall, the data show that across time, female rats display a larger increase in approach behavior, and this effect was larger in adolescents. The analysis of adolescents revealed a significant interaction between sex, treatment, and time (F(1,40) = 8.68; p = .005). The post-hoc analyses revealed that adolescent females exposed to nicotine vapor displayed a greater increase in nosepokes over time than female controls (*p ≤ .05). Also, female adolescents that were exposed to nicotine displayed more nosepoke responses on Day 14 as compared to Day 1 (#p ≤ .05). On Day 14, female adolescent rats that were exposed to nicotine also displayed more nosepoke responses than adolescent female controls (*p ≤ .05) and their adolescent male counterparts (†p ≤ .05). Female adults that were exposed to nicotine displayed more nosepoke responses on Day 14 as compared to Day 1 (#p ≤ .05). On Day 14, female adults that were exposed to nicotine also displayed more nosepoke responses than adult female controls (*p ≤ .05) and their adult male counterparts (†p ≤ .05). Male adults that were exposed to nicotine displayed more nosepoke responses than adult male controls (*p ≤ .05).
Figure 2. Adolescent Females Display a Greater Increase in Approach Behavior Over Time Relative to Day 1.
Note. The data reflect mean (± SEM) nosepoke responses on Day 14 expressed as percent change from Day 1 in female and male adolescent and adult rats. Individual data points are color matched to allow for comparisons in each pair of rats. The asterisks (*) denote a significant difference from controls, the daggers (†) denote a difference from males, and the number signs (#) denote a difference from Day 1 of the exposure regimen (p ≤ .05).
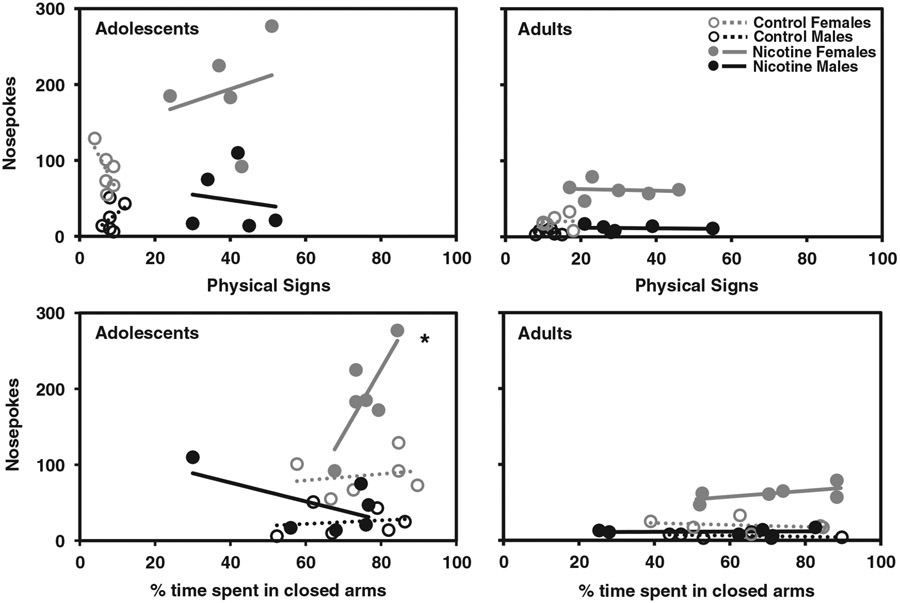
Figure 3 displays correlational analyses between approach behavior and withdrawal-induced changes in physical signs and anxiety-like behavior in control (open circles with dotted lines) and nicotine vapor (closed circles with solid lines) groups on Day 14. Overall, the results revealed that approach behavior was correlated with withdrawal-induced increases in anxiety-like behavior. Specifically, in adolescent females that were exposed to nicotine vapor, nosepoke responses were positively correlated with percent time spent in closed arms (r = 0.80, *p = .05). The nosepoke responses in this group were not correlated with physical signs of withdrawal.
Figure 3. In Adolescent Females, Approach Behavior Is Correlated with Anxiety-Like Behavior.
Note. The data reflect a correlational analysis between nosepoke responses and withdrawal-induced increases in physical signs and anxiety-like behavior, which is noted as percent time spent in the closed arm of the EPM on Day 14. The asterisk (*) denotes a significant correlation between nosepoke responses and percent time spent in the closed arm of the EPM (p ≤ .05).
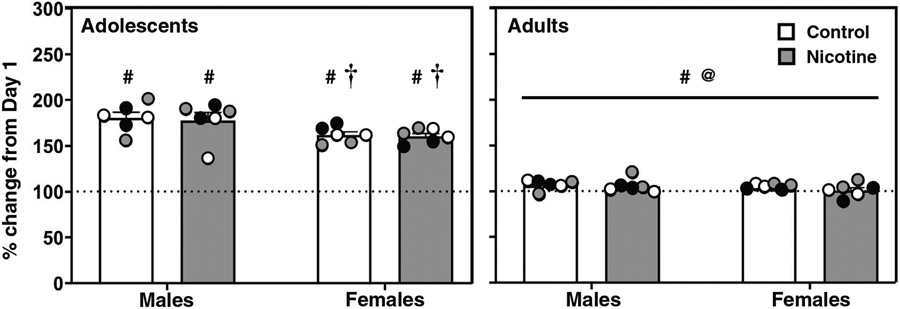
Figure 4 displays changes in body weight in control (white bars) and nicotine vapor (grey bars) groups on Day 14 expressed as percent change from Day 1. Overall, the data show that adolescents gain more weight than adults, and the magnitude of weight gain was greater in males. The analysis of adolescent rats revealed a significant interaction between sex and time (F(1,40) = 6.63; p = .001). Post-hoc analyses revealed that all adolescent rats gained weight across time (#p ≤ .05), an effect that was larger in males (†p ≤ .05). The analysis of adult rats revealed a main effect of time (F(1,40) = 5.77; p = .02), with all adult rats gaining some weight across time (#p ≤ .05). Lastly, there was a larger increase in weight gain in adolescent versus adult rats regardless of their treatment condition (@p ≤ .05).
Figure 4. Adolescent Rats Gain More Weight Over Time than Adults.
Note. The data reflect mean (± SEM) changes in body weight on Day 14 expressed as percent change from Day 1 in female and male adolescent and adult rats. Individual data points are color matched to allow for comparisons in each pair of rats. The daggers (†) denote a significant difference from males, the at sign (@) denotes a difference from adults, and the number signs (#) denote a difference from Day 1 (p ≤ .05).
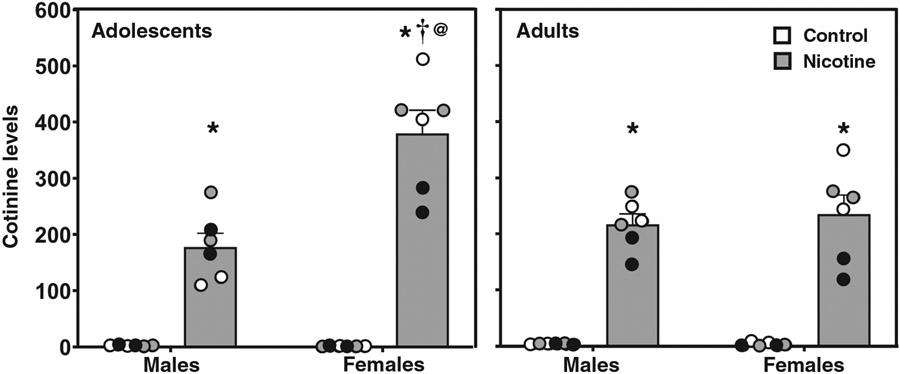
Figure 5 displays cotinine levels in control (white bars) and nicotine vapor (grey bars) groups on Day 14. Overall, the data reveal that adolescent females that were exposed to nicotine vapor displayed the largest increase in cotinine levels at the end of the final exposure session. The overall analysis revealed a significant interaction between sex, age, and treatment (F(1,40) = 8.94; p = .005). All rats that were exposed to nicotine vapor displayed an increase in cotinine levels relative to their respective control group (*p ≤ .05). Adolescent females that were exposed to nicotine vapor displayed higher cotinine levels than adult females (@p ≤ .05) and adolescent males (†p ≤ .05).
Figure 5. Adolescent Females Display Higher Cotinine Levels Following Nicotine Vapor Exposure.
Note. The data reflect mean (± SEM) serum cotinine levels in female and male adolescent and adult rats on Day 14 of the exposure regimen. Individual data points are color matched to allow for comparisons in each pair of rats. The asterisks (*) denote a significant difference from controls, the dagger (†) denotes a difference from males, and the at sign (@) denotes a difference from adults (p ≤ .05).
Discussion
In summary, our major finding was that female rats displayed greater approach behavior to a port that delivered nicotine vapor as compared to males, and this effect was larger in adolescents. In adolescent females, the results also revealed that approach behavior was correlated with withdrawal-induced increases in anxiety-like behavior, but not physical signs of withdrawal. Over time, adolescent rats gained more weight than adults, and this effect was larger in adolescent males regardless of their treatment condition. Not surprisingly, female adolescents that exhibited the largest amount of nosepoke responses in the port that delivered nicotine also displayed the highest levels of the nicotine metabolite, cotinine.
A major finding of the present report was that across time adolescent female rats displayed the largest increase in approach behavior toward the delivery site of the nicotine vapor plumes. The time-dependent increase in the magnitude of nosepoke responses is believed to reflect greater nicotine reward-seeking behavior in adolescent females. This interpretation of our nosepoke data is consistent with previous work comparing age and sex differences in the rewarding effects of nicotine. For example, the magnitude of place preference produced by nicotine is larger in female adolescent rats as compared to their male counterparts (Torres et al., 2008; Torres et al., 2009). Another report using intravenous self-administration procedures revealed that female adolescent rats display two-fold higher levels of nicotine intake relative to female adults (Levin et al., 2007). Adolescent female rats also acquire nicotine self-administration more rapidly and maintain higher levels of nicotine intake than adults (Chen et al., 2007). In a meta-analysis that included all of the existing nicotine self-administration studies in rats, the major conclusion was that the magnitude of nicotine intake is larger in female versus male rats, and the effect size was larger in adolescents (Flores et al., 2019). Thus, the present study supports prior work demonstrating that the reinforcing effects of nicotine are greater in adolescent rats, particularly female adolescents.
The present study extends prior work by showing that the motivational effects of nicotine vapor are stronger in females during the adolescent period of development. Prior reports have compared nicotine vapor self-administration in rodents. One report in adult male mice found that reliable nicotine vapor self-administration required the addition of menthol or flavorants (Cooper et al., 2021). Another report showed stable nicotine vapor self-administration in adult female and male rats, albeit with low discrimination between the active and inactive lever (Smith et al., 2020). Indeed, another report comparing sex differences found that the discriminative stimulus effects of nicotine vapor were lower in females versus males (Lefever et al., 2019). A recent report also revealed that adolescent male rats displayed larger shifts in preference behavior than adult males using a shorter puff duration than was used in the present study (Frie et al., 2020). Another study found that adolescent female mice escalated their consumption of a flavored nicotine solution as compared to adolescent male mice (Patten et al., 2021). Together with existing work, the present findings suggest that the discriminative stimulus and reinforcing effects of nicotine are enhanced in adolescent female rats. It is noted that adolescent female controls displayed higher nosepoke responses for the port that delivered ambient air. In prior work with self-administration procedures, we have noted that adolescent rats display higher responding on an inactive lever as compared to adults (Natividad et al., 2013). Thus, in the present study the nosepoke responses in the air port are believed to reflect hyperactivity in young animals. Importantly, our effects appear to be specific to nicotine given that adolescents displayed higher nosepoke responses for the port that delivered nicotine as compared to air controls, suggesting that our effects were motivated by the rewarding effects of nicotine.
One goal of this report was to provide insight into the role of nicotine dependence in motivating approach behavior in female and male rats from different age groups. The correlational analyses revealed that the magnitude of approach behavior following nicotine vapor exposure was closely associated with the expression of anxiety-like behavior during withdrawal. Interestingly, there was no correlation between approach behavior and physical signs of withdrawal. These findings suggest that adolescent females may be more motivated to seek nicotine following repeated exposure to alleviate negative affective states produced by withdrawal. Prior work in our laboratory has found that withdrawal severity is lower in adolescent versus adult rats that received nicotine via osmotic minipumps (see O’Dell, 2009). Thus, the possibility exists that age differences in withdrawal may vary in procedures involving passive and continuous delivery of nicotine as compared to procedures involving volitional intake of inhaled nicotine vapor.
It is recognized that group differences in body weight have the potential to influence the amount of nicotine that is absorbed. One might expect that following exposure to the same amount of nicotine, a large animal might display lower nicotine levels as compared to a smaller rat due to differences in size. However, in the present study the female adolescent rats displayed higher nosepoke responses and the highest cotinine levels relative to all other groups. The analysis of changes in body weight revealed that adolescent rats gained more weight than adults regardless of treatment. This pattern of results is consistent with a prior self-administration study showing that both female and male adolescent rats gained more weight than adults regardless of their self-administration history (Schassburger et al., 2016). Thus, group differences in body weight do not likely explain the pattern of results in nosepoke responses that were age- and sex-dependent.
The present study also confirms that female adolescents that displayed the largest increase in nosepoke responses in the port that delivered nicotine, also displayed the highest cotinine levels. Since cotinine is a direct metabolite of nicotine with a longer half-life, the assessments of cotinine served as a biomarker for detecting different levels of nicotine intake across treatment groups. The increased cotinine levels observed in rats that spent more time near the vapor input port is likely driven by higher nicotine intake through inhalation, and in part, through oral and transdermal absorption. Prior work using osmotic pumps has shown that delivery of the same concentration of nicotine results in lower cotinine levels in adolescent rats, suggesting that nicotine metabolism is faster during adolescence (Torres et al., 2013; Trauth et al., 2000). There are mixed results regarding sex differences in nicotine metabolism following exposure to nicotine via osmotic pumps (Torres et al., 2013) versus inhalation (Lallai et al., 2021) procedures. Thus, more work is needed to better assess the impact of age and sex on nicotine metabolism.
The present work lays a foundation for ongoing efforts in our laboratory to establish nicotine vapor self-administration. Future studies will utilize a two-phase procedure whereby the rats will first receive passive delivery of nicotine vapor before the animals are allowed to nosepoke for additional nicotine plumes. Based on the present findings, it is expected that the rats will readily self-administer nicotine vapor given that over time they display an increase in nosepokes in the port that delivered the nicotine plumes. It is also expected that females will display the quickest acquisition of instrumental responding and the highest level of self-administration behavior, particularly if the second phase of the study is initiated during adolescence. Our predictions are supported by prior work showing that female rats display greater approach responses to nicotine-predictive cues than males (Stringfield et al., 2019). The present work is an important first step in our long-term goal of elucidating the mechanisms that modulate age and sex differences in nicotine use.
Acknowledgments
The present study was supported by the National Institute on Drug Abuse DA021274 and DA033613 to LEO; DA052119 to IAM and the Brain and Behavior Research Foundation Young Investigator Award (24994 to IAM). Isabella Liano was supported by funding from the On Campus Student Employment Opportunities at UTEP through the COURI-MERITUS program.
References
- Chen H, Matta SG, & Sharp BM (2007). Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology, 32(3), 700–709. 10.1038/sj.npp.1301135 [DOI] [PubMed] [Google Scholar]
- Chen JC, Das B, Mead EL, & Borzekowski DLG (2017). Flavored e-cigarette use and cigarette smoking susceptibility among youth. Tobacco Regulatory Science, 3(1), 68–80. 10.18001/trs.3.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SY, Akers AT, & Henderson BJ (2021). Flavors enhance nicotine vapor self-administration in male mice. Nicotine & Tobacco Research, 23(3), 566–572. 10.1093/ntr/ntaa165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF (2016). A longitudinal study of cotinine in long-term daily users of e-cigarettes. Drug and Alcohol Dependence, 160, 218–221. 10.1016/j.drugalcdep.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Flores RJ, Alshbool FZ, Giner P, O’Dell LE, & Mendez IA (2022). Exposure to nicotine vapor produced by an electronic nicotine delivery system causes short-term increases in impulsive choice in adult male rats. Nicotine & Tobacco Research, 24(3), 358–365. 10.1093/ntr/ntab141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores RJ, Uribe KP, Swalve N, & O’Dell LE (2019). Sex differences in nicotine intravenous self-administration: A meta-analytic review. Physiology & Behavior, 203, 42–50. 10.1016/j.physbeh.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flouris AD, Chorti MS, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, Wallace Hayes A, Tsatsakis AM, & Koutedakis Y (2013). Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhalation Toxicology, 25(2), 91–101. 10.3109/08958378.2012.758197 [DOI] [PubMed] [Google Scholar]
- Frie JA, Underhill J, Zhao B, de Guglielmo G, Tyndale RF, & Khokhar JY (2020). OpenVape: An open-source e-cigarette vapor exposure device for rodents. Eneuro, 7(5), ENEURO.0279–20. 10.1523/eneuro.0279-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Grieder TE, Cole M, & Koob GF (2010). Exposure to chronic intermittent nicotine vapor induces nicotine dependence. Pharmacology Biochemistry and Behavior, 96(1), 104–107. 10.1016/j.pbb.2010.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Whitaker AM, Baynes B, Abdel AY, Weil MT, & George O (2014). Nicotine vapor inhalation escalates nicotine self-administration. Addiction Biology, 19(4), 587–592. 10.1111/adb.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giner P, Maynez-Anchondo L, Liley AE, Uribe KP, Frietze GA, Simon NW, & Mendez IA (2022). Increased risky choice and reduced CHRNB2 expression in adult male rats exposed to nicotine vapor. International Journal of Molecular Sciences, 23(3), 1231. 10.3390/ijms23031231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi-Paydar M, Kerr TM, Harvey EL, Cole M, & Taffe MA (2019). Effects of nicotine and THC vapor inhalation administered by an electronic nicotine delivery system (ENDS) in male rats. Drug and Alcohol Dependence, 198, 54–62. 10.1016/j.drugalcdep.2019.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, de Guglielmo G, Larrosa E & George O (2019). Exposure to passive nicotine vapor in male adolescent rats produces a withdrawal-like state and facilitates nicotine self-administration during adulthood. European Neuropsychopharmacology, 29(11), 1227–1234. 10.1016/j.euroneuro.2019.08.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BA, Patel R, Nguyen KH, & Dube SR (2015). Trends in awareness and use of electronic cigarettes among US adults, 2010-2013. Nicotine & Tobacco Research, 17(2), 219–227. 10.1093/ntr/ntu191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, & Krishnan-Sarin S (2017). A call to end the epidemic of adolescent E-cigarette use. Drug and Alcohol Dependence, 174, 215–221. 10.1016/j.drugalcdep.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallai V, Chen YC, Roybal MM, Kotha ER, Fowler JP, Staben A, Cortez A, & Fowler CD (2021). Nicotine e-cigarette vapor inhalation and self-administration in a rodent model: Sex- and nicotine delivery-specific effects on metabolism and behavior. Addiction Biology, 26(6), e13024. 10.1111/adb.13024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefever TW, Lee YOK, Kovach AL, Silinski MAR, Marusich JA, Thomas BF, & Wiley JL (2017). Delivery of nicotine aerosol to mice via a modified electronic cigarette device. Drug and Alcohol Dependence, 172, 80–87. 10.1016/j.drugalcdep.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefever TW, Thomas BF, Kovach AL, Snyder RW, & Wiley JL (2019). Route of administration effects on nicotine discrimination in female and male mice. Drug and Alcohol Dependence, 204, 107504. 10.1016/j.drugalcdep.2019.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, & Slotkin TA (2007). Adolescent vs. adult-onset nicotine self-administration in male rats: Duration of effect and differential nicotinic receptor correlates. Neurotoxicology and Teratology, 29(4), 458–465. 10.1016/j.ntt.2007.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanari C, Kelley LK, Kerr TM, Cole M, & Gilpin NW (2020). Nicotine e-cigarette vapor inhalation effects on nicotine & cotinine plasma levels and somatic withdrawal signs in adult male Wistar rats. Psychopharmacology, 237(3), 613–625. 10.1007/s00213-019-05400-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2010). Guide for the care and use of laboratory animals. National Academies Press. https://scholar.google.com/scholar_lookup?title=Guide+for+the+care+and+use+of+laboratory+animals&publication_year=2010& [Google Scholar]
- Natividad LA, Torres OV, Friedman TC, & O’Dell LE (2013). Adolescence is a period of development characterized by short- and long-term vulnerability to the rewarding effects of nicotine and reduced sensitivity to the anorectic effects of this drug. Behavioural Brain Research, 257, 275–285. 10.1016/j.bbr.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE (2009). A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neuropharmacology, 56 Suppl 1(Suppl 1), 263–278. 10.1016/j.neuropharm.2008.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang RD, Goldenson NI, Kirkpatrick M, Barrington-Trimis JL, Cho J, & Leventhal AM (2020). Sex differences in the appeal of flavored e-cigarettes among young adult e-cigarette users. Psychology Addiction Behavior, 34(2), 303–307. 10.1037/adb0000548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten T, Dreier A, Herman RJ, Kimball BA, & De Biasi M (2021). Exposure to fruit-flavoring during adolescence increases nicotine consumption and promotes dose escalation. Neuropharmacology, 195, 108672. 10.1101/2021.01.12.426210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper JK, & Brewer NT (2014). Electronic nicotine delivery system (electronic cigarette) awareness, use, reactions, and beliefs: A systematic review. Tobacco Control, 23(5), 375–384. 10.1136/tobaccocontrol-2013-051122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzoni L, Moretti M, Sala M, Fasoli F, Mucchietto V, Lucini V, Cannazza G, Gallesi G, Castellana CN, Clementi F, Zoli M, Gotti C & Braida D (2015). Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. European Neuropsychopharmacology, 25(10), 1775–1786. 10.1016/j.euroneuro.2015.06.010 [DOI] [PubMed] [Google Scholar]
- Schassburger RL, Pitzer EM, Smith TT, Rupprecht LE, Thiels E, Donny EC, & Sved AF (2016). Adolescent rats self-administer less nicotine than adults at low doses. Nicotine & Tobacco Research, 18(9), 1861–1868. 10.1093/ntr/ntw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LC, Kallupi M, Tieu L, Shankar K, Jaquish A, Barr J, Su Y, Velarde N, Sedighim S, Carrette LLG, Klodnicki M, Sun X, De Guglielmo G, & George O (2020). Validation of a nicotine vapor self-administration model in rats with relevance to electronic cigarette use. Neuropsychopharmacology, 45(11), 1909–1919. 10.1038/s41386-020-0734-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfield SJ, Madayag AC, Boettiger CA & Robinson DL (2019). Sex differences in nicotine-enhanced Pavlovian conditioned approach in rats. Biology of Sex Differences, 10(1), 37. 10.1186/s13293-019-0244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres O, Tejeda H, Natividad L, & O’Dell LE (2008). Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacology Biochemistry and Behavior, 90(4), 658–663. 10.1016/j.pbb.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Gentil LG, Natividad LA, Carcoba LM & O’Dell LE (2013). Behavioral, biochemical, and molecular indices of stress are enhanced in female versus male rats experiencing nicotine withdrawal. Frontiers in Psychiatry, 4, 38. 10.3389/fpsyt.2013.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, & O’Dell LE (2009). Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology, 206(2), 303–312. 10.1007/s00213-009-1607-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Pipkin JA, Ferree P, Carcoba LM, & O’Dell LE (2015). Nicotine withdrawal increases stress-associated genes in the nucleus accumbens of female rats in a hormone-dependent manner. Nicotine & Tobacco Research, 17(4), 422–430. 10.1093/ntr/ntu278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, & Slotkin TA (2000). Persistent and delayed behavioral changes after nicotine treatment in adolescent rats. Brain Research, 880(1-2), 167–172. 10.1016/s0006-8993(00)02823-7 [DOI] [PubMed] [Google Scholar]
- Zhu SH, Gamst A, Lee M, Cummins S, Yin L, & Zoref L (2013). The use and perception of electronic cigarettes and snus among the U.S. population. PLOS ONE, 8(10), e79332. 10.1371/journal.pone.0079332 [DOI] [PMC free article] [PubMed] [Google Scholar]