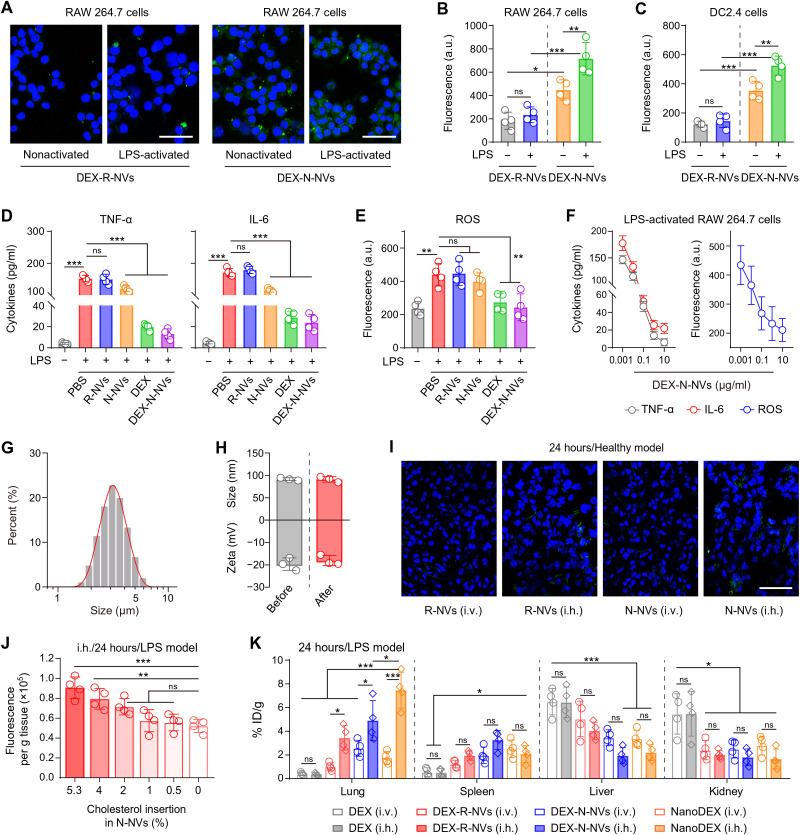
Fig. 3. The nanoDEX showed enhanced targeting to inflamed cells and improved retention in inflamed lungs.
(A) Fluorescence images of nonactivated or LPS-activated RAW 264.7 cells after incubation with DEX-R-NVs or DEX-N-NVs. Scale bars, 100 μm. DEX-R-NVs and DEX-N-NVs are labeled with 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) (green) before the incubation. (B and C) Fluorescence intensity analysis of DiO (green) in (B) RAW 264.7 and (C) DC2.4 cells after indicated treatments. (D) TNF-α, IL-6, and (E) ROS levels in the supernatant of LPS-stimulated RAW 264.7 cells after indicated treatments. (F) Dose-dependent effects of DEX-N-NVs on TNF-α, IL-6, and ROS expression in the supernatant of LPS-stimulated RAW 264.7 cells. (G) Size distribution of the droplets containing DEX-N-NVs after inhalation delivery. (H) Hydrodynamic diameter and zeta potential of DEX-N-NVs before and after inhalation delivery. (I) Fluorescent images showing the lung accumulation of R-NVs or N-NVs after intravenous or inhalation delivery. Scale bars, 50 μm. R-NVs and N-NVs are labeled with DiO (green) before inhalation delivery. (J) Lung accumulation of cholesterol-engineered N-NVs at 24 hours after inhalation delivery in LPS-infected mice. (K) Biodistribution of DEX, DEX-R-NVs, DEX-N-NVs, or nanoDEX in major organs at 24 hours after intravenous or inhalation delivery in LPS-infected mice. All data are presented as means ± SD (n = 4). Statistical significance was calculated via ordinary one-way ANOVA with Tukey’s test (B, C, and K) or Dunnett’s test (D, E, and J). *P < 0.05, **P < 0.01, and ***P < 0.001. a.u., arbitrary units; i.h., inhalation; i.v., intravenous.