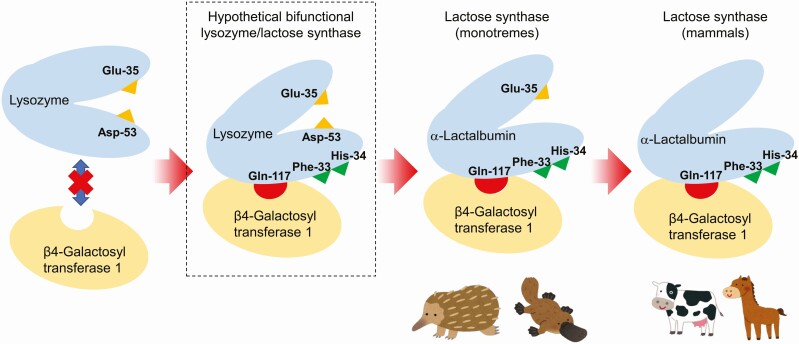
Figure 1.
Schematic picture of a hypothetical scenario of molecular evolution from lysozyme to α-lactalbumin in complex with β4-galactosyl transferase 1 (lactose synthase). Based on the observation that monotremes maintain Glu-35, a catalytic residue of lysozymes, it is hypothesized that acquisition of amino acid residues such as Gln-117 (hydrogen bonding to β4-galactosyl transferase 1), Phe-33 (forming the hydrophobic pocket), and His-34 (forming the hydrophobic pocket, hydrogen bounding to donor glucose) had occurred before entire loss of catalytic activity of lysozyme. From this point of view, presence of a hypothetical bifunctional lysozyme/lactose synthase can be predicted, but no clear evidence has yet found to date.