Abstract
The bed nucleus of the stria terminalis (BNST) is a forebrain region implicated in aversive responses to uncertain threat. Much of the work on the role of BNST in defensive behavior has used Pavlovian paradigms in which the subject reacts to aversive stimuli delivered in a pattern determined entirely by the experimenter. Here, we explore the contribution of BNST to a task in which subjects learn a proactive response that prevents the delivery of an aversive outcome. To this end, male and female rats were trained to shuttle during a tone to avoid shock in a standard two-way signaled active avoidance paradigm. Chemogenetic inhibition (hM4Di) of BNST attenuated the expression of the avoidance response in male but not female rats. Inactivation of the neighboring medial septum in males produced no effect on avoidance, demonstrating that our effect was specific to BNST. A follow up study comparing hM4Di inhibition to hM3Dq activation of BNST in males replicated the effect of inhibition and demonstrated that activation of BNST extended the period of tone-evoked shuttling. These data support the novel conclusion that BNST mediates two-way avoidance behavior in male rats and suggest the intriguing possibility that the systems underlying proactive defensive behavior are sex-specific.
Subject terms: Learning and memory, Emotion
Introduction
An avoidant style of coping is a unifying behavioral feature of many forms of pathological anxiety [1]. Although healthy proactive avoidance responses prevent harm and reduce contact with stressors [2, 3], avoidant behavior that occurs at a relatively low cost can easily become excessive, fostering the maladaptive sensitivity to false alarm prevalent in anxiety disorders [4, 5]. Despite the crucial role of avoidance in both adaptive defense and pathological anxiety, its neural circuitry has not been elucidated.
Two-way signaled active avoidance (SAA) is an acquired form of proactive defensive behavior in rodents. SAA involves a response (two-way shuttling) triggered by a conditioned stimulus (CS, tone) that serves to prevent contact with an aversive unconditioned stimulus (US, shock). As the response is acquired over the course of SAA training, US frequency decreases substantially. Viewed from the perspective of threat imminence theory [6–9], this change in US density transforms the shock from a certain/imminent threat during initial acquisition to a possible/distal threat once the subject reaches asymptotic levels of SAA expression. Indeed, the CS will evoke high-imminence defensive reactions early in SAA training (freezing, ultrasonic vocalization, conditioned suppression, and conditioned analgesia), but these responses decrease as the expression of avoidance becomes more robust [10–15]. Despite evidence for a shift away from the defensive mode evoked by imminent threat, no previous research has explored the role played by the neural substrates of possible/distal threat in SAA.
Prior work demonstrates that conditioned reactions to uncertain threat are underpinned by the bed nucleus of the stria terminalis (BSNT) [16–20]. We therefore hypothesized that the BNST mediates the expression of SAA. To test this hypothesis, we used a chemogenetic approach to manipulate BNST activity in male and female rats performing this behavior. We demonstrated that inhibition of BNST attenuated the expression of the two-way avoidance response in males. However, the same manipulation had no effect on avoidance in females, suggesting that sex-specific circuits underlie SAA. These data establish BNST as a crucial node in the neural system mediating proactive defense in male but not female rats.
Materials & methods
Animals
Subjects were 80 adult Sprague Dawley rats (64 males, 16 females weighing a minimum of either 300 g or 200 g, respectively, at the outset of experimentation). Rats were singly housed and maintained on a 14:10 light/dark cycle with ad libitum access to food and water in the vivarium in the Department of Psychological and Brain Sciences at Texas A&M University. Subjects were briefly handled by experimenters three times on separate days prior to behavioral training. All procedures were conducted during the light phase and with the approval of the Texas A&M IACUC.
Surgery
Animals were anesthetized with a 1 mL/kg dose of a 10:1 mixture of ketamine (100 mg/mL) and xylazine (100 mg/mL) and mounted to a stereotaxic frame. Following incision, bilateral microinjections were made in BNST (ML ± 1.2, AP –0.12 or –0.24, DV –7.3 or –7.6) or a unilateral microinjection was made in MS (ML ± 2.0, AP + 0.36, DV -7.3 with cannula angled at 15 degrees off vertical). All infusions were made with a Hamilton NeurOs syringe (5 μL total capacity, 33-gauge needle) fixed to the carrier arm of the sterotaxis. Using a manual stereotaxic infuser (Stoetling), virus was injected at a rate of 0.1 μL/min for a total of 0.3 μL/injection and were followed by 5 min for diffusion. Each infusion contained an adeno-associated virus (AAV) from Addgene, bearing the gene construct for either an inhibitory or excitatory DREADD (Designer Receptor Exclusively Activated by Designer Drugs [21]) or GFP. The following AAVs, concentrated at 1.2 × 1013 GC/ml, were used: AAV5-hSyn-hM4Di-mCherry, AAV5-hSyn-hM3Dq-mCherry, or AAV5-hSyn-EGFP, depending on the experiment. Animals were allowed at least 28 days of recovery before slice physiology or behavioral training.
Electrophysiological recordings
Coronal brain slices (250 μm) containing the BNST were taken from 6 rats expressing hM4Di, hM3Dq, or GFP in BNST neurons. Slices were positioned in a perfusion chamber attached to the fixed stage of an upright microscope (Olympus) and submerged in continuously flowing oxygenated Artificial cerebrospinal fluid (aCSF) (in mm: 125 NaCl, 4.5 KCl, 2 CaCl2, 1.25 NaH2PO4, 25 NaHCO3, 15 sucrose and 15 glucose) at 32°C. Neurons were viewed under a water-immersion lens (40×) and a CCD camera. A Multiclamp 700B amplifier with Clampex 10.6 software and Digidata1555A (Molecular Devices) were used for recordings. The patch pipettes with resistance 3-5 MΩ were pulled by the pipette puller (Sutter Instrument Co., Model P-97). To measure spontaneous action potential (sAP), cell-attached voltage-clamp recordings of GFP or mCherry-positive putative GABAergic neurons in BNST were performed. Pipette was filled with the K+-based intracellular solution (in mM: 123 potassium gluconate, 10 HEPES, 0.2 EGTA, 8 NaCl, 2 MgATP and 0.3 NaGTP, pH 7.3, 280 mOsm). To record spontaneous EPSCs (sEPSCs), Picrotoxin (100 μM) and D-APV (25 μM) were added into the standard aCSF, and the neuron was held at a potential of –70 mV. Pipette was filled with the Cs-based intracellular solution (in mM: 119 CsMeSO4, 8 TEA. Cl, 15 HEPES, 0.6 EGTA, 5 QX-314.Cl, 7 phosphocreatine, 4 MgATP, 0.3 Na3GTP, 0.1 leupeptin, pH 7.3, 280 mOsm).
Two-way signaled active avoidance (SAA) behavior
Training
Subjects were trained to avoid by shuttling in either direction across a divided chamber during presentation of an auditory CS (15-s, 2-kHz, 70-db pure tone). This response caused the immediate inactivation of the CS and the omission of the US (0.5-s, 0.7-mA scrambled footshock).
Each day of SAA training began with a 5-min acclimation period. To establish the CS-US relationship, the first trial on the first day was a Pavlovian trial, in which a CS-US pairing was delivered regardless of whether the subject shuttled. All other trials included the avoidance contingency (i.e. shuttling during the CS caused CS inactivation and US omission). 30 avoidance trials were presented on each day of SAA training. All trials were separated by a variable duration inter-trial interval that averaged 120 s.
Poor avoider criterion
Between training and test, data from all subjects in all experiments were reviewed and a poor avoidance criterion was applied [10, 14, 22]. Animals averaging six or fewer responses during the final two sessions of training were determined to be poor avoiders. The brains of poor avoiders were visually examined for any obvious lesion, but the extent of viral expression was not characterized.
Test under training conditions
This procedure involved two additional days of two-way SAA with all training parameters in place. The only distinction between training and test was that ip injection of either CNO or vehicle was delivered in a counterbalanced order prior to each test session.
Test under extinction conditions
To create a uniform behavioral assay with common conditions, this test was comprised of a single session in which 10 CSs were presented, each separated by a 2-min ITI. Unlike training, each CS continued for the full 15 s regardless of whether or not the subject shuttled. No USs were presented. All subjects received ip injection of CNO or vehicle (for poor avoiders only) prior to test.
DREADD agonist
Clozapine-N-oxide (CNO; 3 mg/kg/mL) was dissolved in physiological saline with 10% DMSO was administered via intraperitoneal (ip) injection 20-25 min before test. Vehicle injection was physiological saline with 10% DMSO, also administered ip.
Perfusion and viral expression
At the end of each behavioral experiment, animals were deeply anesthetized and transcradially perfused with 10% buffered Formalin. Brains were removed and sliced into 40-μm sections using a cryostat. Sections were mounted onto subbed slides and cover-slipped with Fluoromount (Sigma Aldrich) before examination under a fluorescent microscope (Olympus U-RFL-T microscope with an Olympus DP72 digital color camera and a Pior OptiScan II control system) to reveal either mCherry or GFP expression.
Behavioral analyses
Shuttling data were collected automatically by the Coulbourn GraphicState software used to automate the delivery of stimuli during SAA training and test. Digital videos of all test sessions were also recorded. Using Noldus Ethovision software, these recordings were analyzed offline for freezing and locomotor activity data. Freezing was defined as a bout of inactivity (defined as activity less than 0.1%) lasting longer than 1 sec. Locomotor activity was defined as distance traveled by the center point of each subject.
Figures
Graphs were made using GraphPad Prism. Viral expression images were made with maps from the Swanson atlas [23] using Adobe Illustrator. Behavioral schematics were made using BioRender.com.
Results
Chemogenetic inhibition of BNST attenuates the expression of SAA in male rats
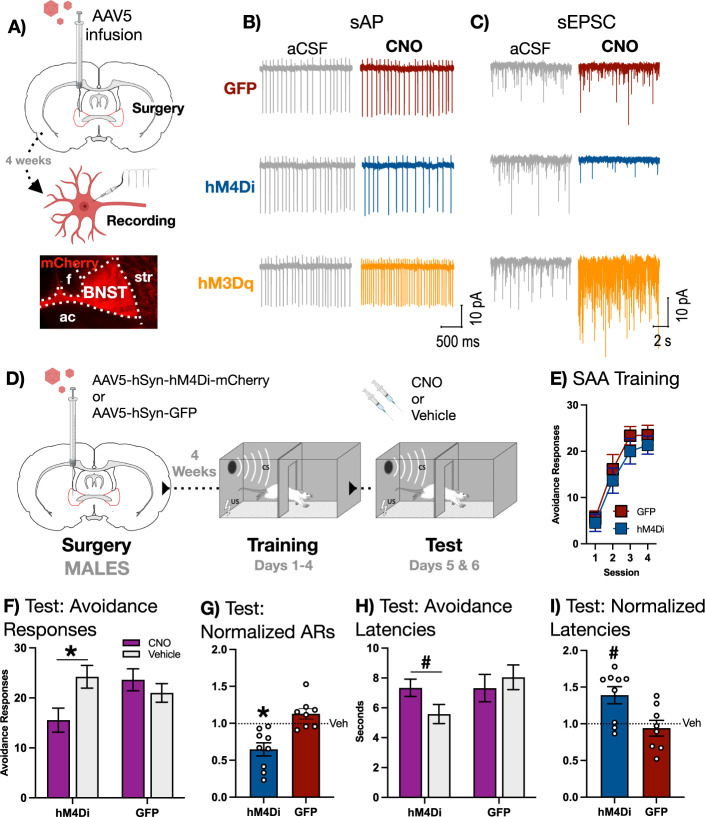
For our initial experiment, we set out to determine the effect of chemogenetic inactivation of BNST on the expression of the avoidance response in male rats. In order to provide a qualitative confirmation of the expected effect of chemogenetic manipulations on neural activity in BNST, we first expressed the inhibitory hM4Di DREADD, the excitatory hM3Dq DREADD, or GFP in the BNST of male rats (procedure schematized in Fig. 2A) and prepared slices from 6 rats (1 rat for each trace presented). Because it has been reported that a subset of neurons in BNST are spontaneously active [24], we performed patch-clamp recordings of spontaneous neural activity in fluorescent cells (mCherry for hM4Di and hM3Dq, or GFP). Baseline cell-attached recordings were made before exposing the slice to the DREADD agonist clozapine-N-oxide (CNO). Bath application of CNO in aCSF had no observable effect on spontaneous action potentials (sAPs) in BNST neurons expressing GFP (Fig. 2B, top). In contrast, CNO decreased the frequency of sAPs in hM4Di-expressing neurons (Fig. 2B, middle) and increased the frequency of sAPs in hM3Dq-expressing neurons (Fig. 2B, bottom). CNO did not change excitatory postsynaptic currents (EPSCs) relative to baseline in GFP-expressing neurons (Fig. 2C, top), though it did result in decreased frequency and amplitude of EPSCs in hM4Di neurons (Fig. 2C, middle) as well as increased frequency and amplitude of EPSCs in hM3Dq neurons (Fig. 2C, bottom). It is worth noting that the observed stimulatory effect of hM3Dq on EPSCs is inconsistent with a previous report, which describes an endocannabinoid-mediated depression in EPSCs caused by hM3Dq activation of BNST neurons [25]. This discrepancy may be attributable to the fact that our data were collected from spontaneously active BNST neurons, one of five discernable physiological types of neuron in BNST [24, 26]. It is possible that hM3Dq activation has an idiosyncratic effect on EPSCs in spontaneously active neurons, reflecting some specific feature of these cells that does not generalize to other types within the BNST. Despite discrepant EPSC results, the effect of both hM4di and hM3dq engagement on action potentials was indeed consistent with prior data [25], suggesting that, in our hands, hM4Di and hM3Dq receptors produced directionally opposite effects on neural activity in BNST.
Fig. 2. BNST is necessary for the expression of two-way avoidance in MALE rats.
A Top: Male subjects received intra-BNST infusions of AAV bearing the gene construct for either hM4Di, hM3Dq, or GFP and allowed to recover before recordings of spontaneous neural activity were conducted in slice. Bottom: representative image of mCherry expression in BNST (ac = anterior commissure, f = fornix, str = striatum). B Cell-attached recordings of spontaneous action potentials (sAPs) in BNST neurons expressing GFP (top), hM4Di (middle), or hM3Dq (bottom). Bath application of 10 μm of CNO had no effect on GFP-expressing neurons, but caused a decrease in sAPs in hM4Di-expressing neurons and an increase in hM3Dq-expressing neurons. C Representative whole-cell recordings of spontaneous excitatory synaptic currents (sEPSCs) in BNST neurons expressing GFP (top), hM4Di (middle), or hM3Dq (bottom). Bath application of 10 μm of CNO had no effect on GFP-expressing neurons, but caused a decrease in frequency and amplitude of sEPSCs in hM4Di-expressing neurons and an increase in hM3Dq-expressing neurons. D Male subjects received intra-BNST infusions of AAV bearing the gene construct for either the hM4Di DREADD or GFP and allowed to recover. Following recovery, subjects underwent 4 days SAA training prior to a pair of tests conducted under training conditions, preceded by either CNO or vehicle. F SAA training data. E Avoidance responses at test: CNO significantly attenuated expression of the two-way avoidance response in hM4di- but not GFP-expressing subjects (* signifies p < 0.01). G Avoidance responses at test normalized to vehicle: CNO decreased the expression of avoidance in the hM4Di group relative to the GFP group (* signifies p < 0.01). H Avoidance latencies at test: CNO significantly increased the latency to avoid in hM4Di- but not GFP-expressing subjects (# signifies p < 0.05). I Avoidance latencies at test normalized to vehicle: CNO significantly increased avoidance latency in the hM4Di group relative to the GFP group (# signifies p < 0.05). All behavioral graphs depict mean (±SEM).
Next, we expressed the inhibitory hM4Di DREADD or GFP in the BNST of male rats (Fig. 1A left is a representative example of mCherry fluorescence in males, Fig. 1B depicts viral expression in both hM4Di and GFP groups) that received four days of SAA training following recovery from surgery. At the end training but before test, two rats were determined to be poor avoiders and were removed from our analysis, leaving us with the following groups: hM4Di (n = 9) and GFP (n = 8). These groups then underwent a pair of tests in which two normal SAA training sessions were preceded by either CNO or vehicle given in a counterbalanced order (design schematized in Fig. 2D).
Fig. 1. Viral expression in all subjects.
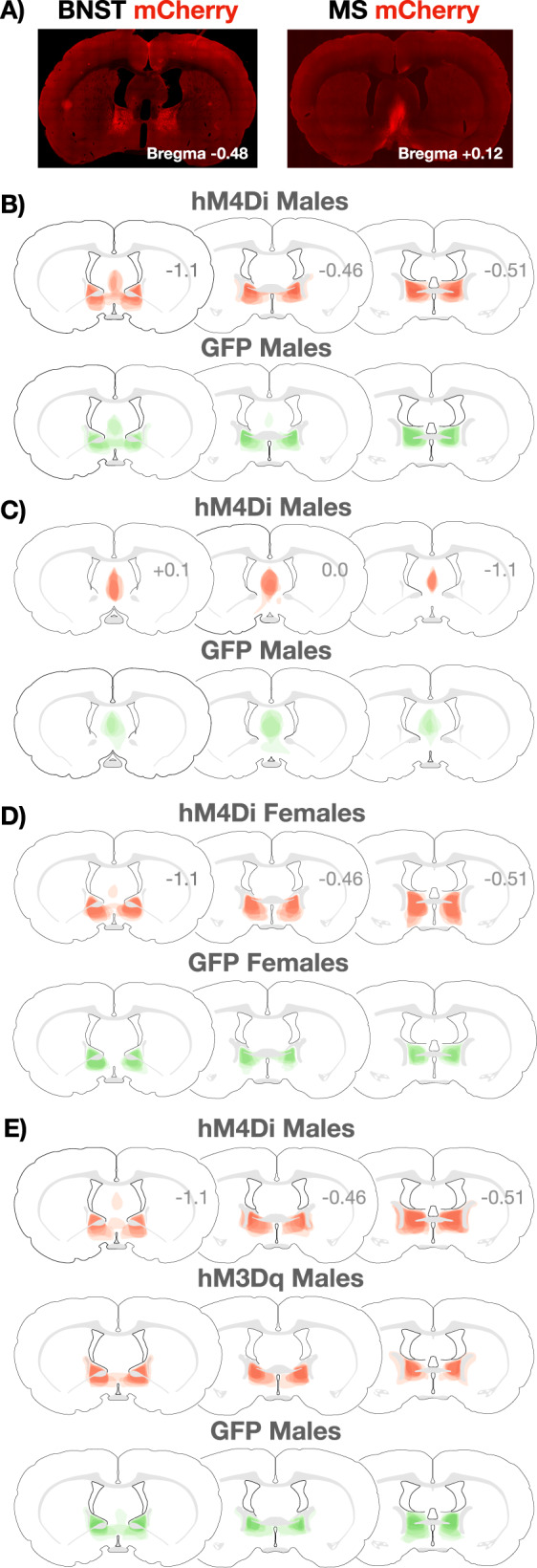
A Representative images of mCherry expression in the BNST (left) and MS (right). B Extent of viral expression in MALE hM4Di (mCherry, top) and GFP (bottom) subjects from the experiment in which BNST was inactivated with CNO during a test conducted under training conditions (see Fig. 2). C Extent of viral expression in MALE hM4Di (mCherry, top) and GFP (bottom) subjects from the experiment in which MS was inactivated with CNO during a test conducted under training conditions (see Fig. 3). D Extent of viral expression in FEMALE hM4Di (mCherry, top) and GFP (bottom) subjects from the experiment in which BNST was inactivated with CNO during a test conducted under training conditions (see Fig. 4). E Extent of viral expression in MALE hM4Di (mCherry, top), hM3Dq (mCherry, middle), and GFP (bottom) subjects from the experiment in which BNST was activated or inactivated with CNO during a test conducted under extinction conditions (see Fig. 5).
Both hM4Di and GFP groups acquired SAA (Fig. 2E). At test, CNO attenuated the expression of the avoidance responses in subjects expressing hM4Di but not GFP (Fig. 2F). A two-way, mixed-design ANOVA with a within-subjects factor of Drug (CNO or vehicle) and a between-subjects factor of Group (hM4Di or GFP) revealed a significant Drug X Group interaction [F(1,15) = 15.91, p = 0.0012]. Fisher’s LSD post-hoc tests further revealed that this interaction was driven by a significant reduction in avoidance responses when the hM4Di group received CNO relative to vehicle (p = 0.0004). No such reduction was observed in the GFP group. To further examine the effects of BNST inactivation on avoidance, we normalized behavior during test by dividing each subject’s CNO avoidance responses by vehicle avoidance responses (Fig. 2G). Two-tailed t test conducted on these data confirmed a significant decrease in normalized avoidance responses in hM4Di relative to GFP subjects [t(15) = 4.185, p = 0.0008]. Thus, chemogenetic inhibition of BNST attenuated the expression of SAA.
Because hM4Di subjects displayed residual avoidance despite the influence of BNST inactivation, we measured the latency to avoid during test to determine whether these remaining responses were altered by inhibition of BNST. Administration of CNO increased avoidance latencies in subjects expressing hM4Di but not GFP (Fig. 2H). A two-way, mixed-design ANOVA with a within-subjects factor of Drug (CNO or vehicle) and a between-subjects factor of Group (hM4Di or GFP) revealed a significant Drug X Group interaction [F(1,15) = 5.529, p = 0.033]. Fisher’s LSD post-hoc test further revealed that this interaction was driven by a significant increase in avoidance latency when hM4Di-expressing subjects received CNO relative to vehicle (p = 0.028). To further explore the influence of BNST inactivation on the latency to avoid, we normalized each subject’s latencies during test (Fig. 2I). Two-tailed t test performed on these data revealed a significant increase in hM4Di subjects relative to GFP subjects [t(15) = 2.831, p = 0.013]. Thus, BNST inactivation reduced the number of avoidance responses at test and also increased the latency to perform the residual responses that persisted in BNST-inactivated male rats.
Chemogenetic inhibition of the medial septum has no effect on the expression of SAA in male rats
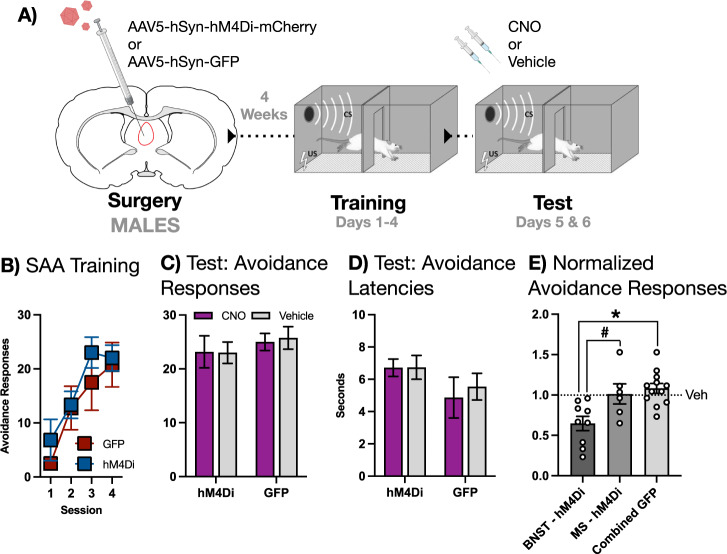
Several subjects in our initial experiment showed mCherry-expression in the medial septum (MS) (Fig. 2B). To control for the effect of hM4Di inactivation of MS on avoidance in males, we performed an experiment in which we explicitly tested the role of MS in SAA. The design of this experiment was identical to the previous, with the exception that hM4Di (n = 6) or GFP (n = 4) were expressed in MS (Fig. 1A right is a representative example of mCherry fluorescence, Fig. 1C depicts viral expression). Subjects received four sessions of SAA training prior to a pair of tests under training conditions preceded by CNO or vehicle in a counterbalanced order (design schematized in Fig. 3A). All subjects acquired SAA during training (Fig. 3B). Two-way mixed-design ANOVA revealed that CNO had no effect on the expression of the avoidance response (Fig. 3C) or on avoidance latencies (Fig. 3D) at test.
Fig. 3. MS is not required for the expression of two-way avoidance in MALE rats.
A Male subjects received intra-MS infusions of AAV bearing the gene construct for either the hM4Di DREADD or GFP and allowed to recover. Following recovery, subjects underwent 4 days SAA training prior to a pair of tests under training conditions, preceded by either CNO or vehicle. B SAA training data. C Avoidance responses at test: CNO had no effect on the expression of the two-way avoidance response. D Avoidance latencies at test: CNO had no effect on the latency to avoid. E Avoidance responses normalized to vehicle in subjects expressing hM4Di in either BNST or MS compared to a combined BNST/MS GFP group. CNO decreased the expression of avoidance in the BNST-hM4Di group relative to the MS-hM4Di group (# signifies p < 0.05) and the combined GFP group (* signifies p < 0.01). All graphs depict mean (±SEM).
Normalized avoidance scores for each subject from this and the previous experiment were then subjected to a direct statistical comparison to establish that our behavioral effects were BNST-specific in males. This analysis revealed that CNO selectively decreased the expression of the avoidance response in the BNST-hM4Di group (Fig. 3E). A one-way ANOVA comparing BNST-hM4Di, MS-hM4Di, and a combined GFP group revealed a significant main effect [F(2,24) = 8.193, p = 0.0019]. Fisher’s LSD post-hoc tests further revealed that this effect was driven by a significant decrease in normalized avoidance in the BNST-hM4Di group relative to both the MS-hM4Di (p = 0.011) and the combined GFP group (p = 0.0006). No significant difference was observed between MS-hM4Di and GFP groups. Thus, we conclude that the effect of CNO on SAA in our hM4Di-expressing male subjects was specific to BNST.
Chemogenetic inhibition of BNST has no effect on the expression of SAA in female rats
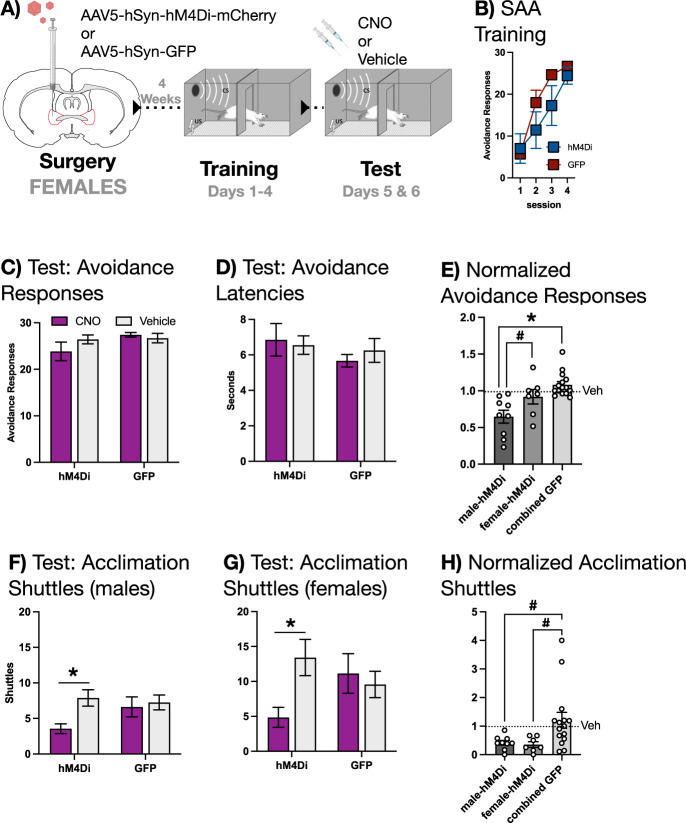
To fully explore the contribution of BNST to SAA, we performed an experiment identical to the one described above for males, but this time in female rats. The inhibitory hM4Di (n = 7) DREADD or GFP (n = 7) were expressed in the BNST (Fig. 1D depicts the extent of viral expression). Subjects received four days of SAA training prior to a pair of tests conducted under training conditions, each of which was preceded by CNO or vehicle (behavioral design schematized in Fig. 4A). Intriguingly, two-way, mixed-design ANOVAs revealed that CNO had no effect on the expression of the avoidance response (Fig. 4C) or on avoidance latencies (Fig. 4D). To verify this sex difference with a direct statistical comparison, normalized avoidance scores from males and females were subjected to a one-way ANOVA (Fig. 4E). This analysis confirmed a significant main effect [F(2,28) = 11.1914, p = 0.0003], and Fisher’s LSD post-hoc tests demonstrated that CNO decreased the expression of avoidance in BNST-hM4Di males relative to both BNST-hM4Di females (p = 0.0196) and a combined GFP group (p < 0.0001).
Fig. 4. BNST is not required for the expression of two-way avoidance in FEMALE rats.
A Female subjects received intra-BNST infusions of AAV bearing the gene construct for either the hM4Di DREADD or GFP and allowed to recover. Following recovery, subjects underwent 4 days SAA training prior to a pair of tests under training conditions, preceded by either CNO or vehicle. B SAA training data. C Avoidance responses at test: CNO had no effect on the expression of the two-way avoidance response. D Avoidance latencies at test: CNO had no effect on the latency to avoid. E Avoidance responses normalized to vehicle in male and female subjects expressing hM4Di in BNST compared to a combined male/female BNST GFP group. CNO decreased the expression of avoidance in the male-hM4Di group relative to the female-hM4Di group (# signifies p < 0.05) and the combined GFP group (* signifies p < 0.01). F Shuttles during acclimation in male subjects (see Fig. 2) at test: CNO decreased shuttling in hM4Di but not GFP subjects (* signifies p < 0.01). G Shuttles during acclimation in female subjects at test: CNO decreased shuttling in hM4Di but not GFP subjects (* signifies p < 0.01). H Shuttles during acclimation normalized to vehicle in male and female subjects expressing hM4Di in BNST compared to a combined male/female BNST GFP group. CNO decreased shuttling during acclimation in the male-hM4Di and female-hM4Di groups compared to the combined GFP group (# signifies p < 0.05). All graphs depict mean (±SEM).
These results caused us to revisit our initial BNST experiment in males to explore whether any behavioral effect of chemogenetic inactivation could be detected in both sexes. Upon examining our data, we hypothesized that unreinforced shuttles during acclimation were, in contrast to avoidance responses, BNST-dependent in both males and females (Fig. 4F, G). Acclimation-shuttling data drawn from our BNST-inactivation study in males were subjected to a two-way, mixed-design ANOVA, with a within-subjects factor of Drug (CNO or vehicle) and a between-subjects factor of Group (hM4Di or GFP). This analysis revealed a significant Drug X Group interaction [F(1,15) = 4.7002, p = 0.0467], which Fisher’s LSD post-hoc tests confirmed was driven by a significant decrease in acclimation shuttles when hM4Di-expressing subjects received CNO relative to vehicle (p = 0.0022). An identical analysis, performed on acclimation-shuttling data from our BNST inactivation experiment conducted in females, yielded a similar, significant result [F(1,12) = 9.7442, p = 0.0088]. This was further confirmed by Fisher’s LSD post-hoc tests that revealed a significant decrease in acclimation responses when hM4Di-expressing subjects received CNO relative to vehicle (p = 0.0029).
We followed up on these tests with a direct comparison of normalized acclimation shuttles in males and females, which confirmed that BNST inactivation attenuated this response in both sexes (Fig. 4H). A one-way ANOVA comparing male-hM4Di, female-hM4Di, and a combined GFP group returned a significant main effect [F(2,28) = 4.2121, p = 0.0252], which Fisher’s LSD post-hoc tests revealed was driven by a significant difference between the combined GFP group and the hM4Di-male group (p = 0.0239) as well as the hM4Di-female group (p = 0.0248). Thus, unreinforced shuttles during the acclimation period are attenuated by BNST inactivation in males and females, suggesting an intra-BNST circuit for these responses common to both sexes. This positive behavioral effect for BNST inactivation in females also hints at a sex difference in the mechanism underlying avoidance responses instead of some more technical effect, such as a sex difference in the efficacy of the hM4Di DREADD.
Chemogenetic activation and inhibition of BNST have opposite effects on the expression of SAA in male rats
Because our focus here is the contribution of BNST to proactive defensive behavior (i.e. responses that alter or prevent aversive outcomes, such as avoidance), and because the previous experiment demonstrated a convincing negative effect for BNST inactivation on avoidance in females, we followed up with a study conducted in males only. This experiment was designed to account for the fact that changes in the expression of avoidance alter the pattern of tones and shocks delivered to subjects. We controlled for this factor by examining the role of the BNST in a test conducted under extinction conditions, which involved the uniform delivery of stimuli across groups. We sought to validate our previous results with chemogenetic inhibition (hM4Di) and also to explore whether chemogenetic activation (hM3Dq) would potentiate the avoidance response.
Either hM4Di, hM3Dq, or GFP were expressed in the BNST of male rats (Fig. 1E depicts the extent of viral expression in all subjects). Subjects then received 6 days of SAA training, after which the poor avoider criterion was applied. There was a sufficient number of poor avoiders to create an independent group instead of disqualifying these subjects from testing. Thus, on the day after SAA training ended, the following groups received an avoidance test conducted under extinction conditions: hM4Di (n = 8), hM3Dq (n = 8), GFP (n = 9), and poor avoiders (n = 6). All subjects received CNO prior to test, with the exception of the poor avoider group, which received vehicle (design schematized in Fig. 5A).
Fig. 5. BNST is necessary for, and sufficient to enhance, the expression of two-way avoidance in MALE rats.
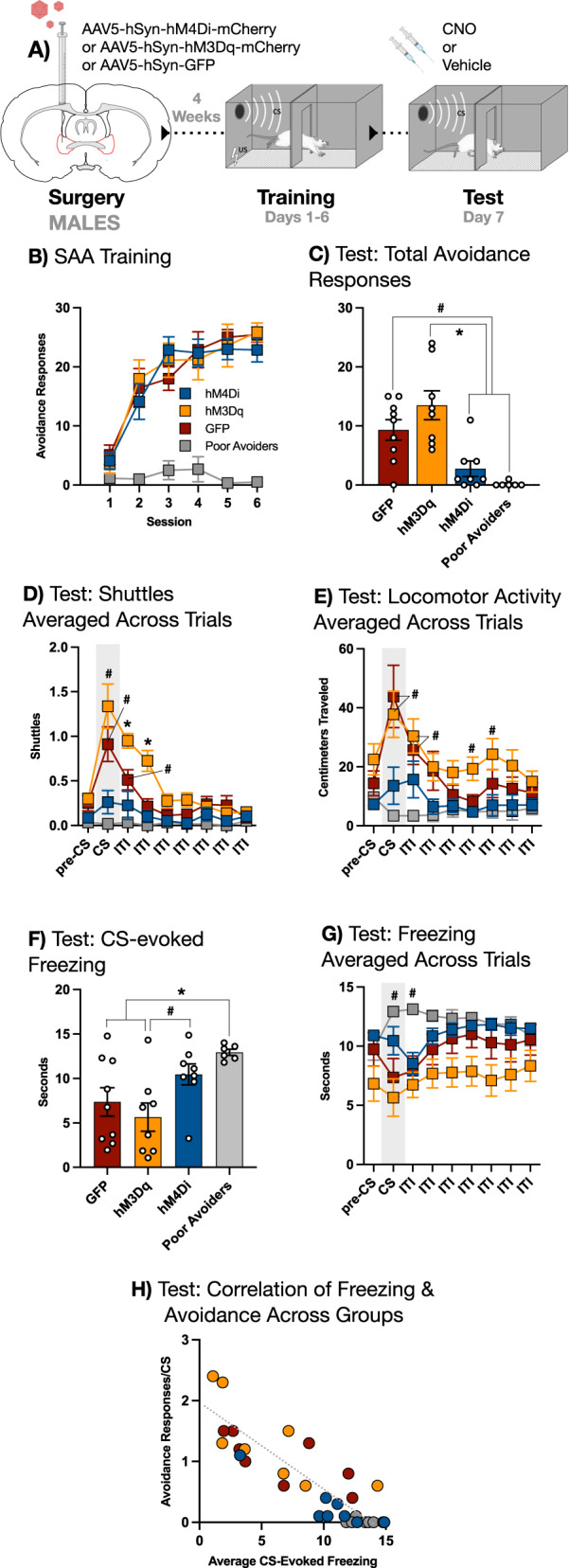
A Male subjects received intra-BNST infusions of AAV bearing the gene construct for either the hM4Di DREADD, the hM3Dq DREADD, or GFP and allowed to recover. Following recovery, subjects underwent 6 days SAA training prior to a single test conducted under extinction conditions (10 CSs, no USs), preceded by either CNO or vehicle (for poor avoiders only). B SAA training data. C Total avoidance responses (shuttles during the CS) at test: GFP controls produced more avoidance responses relative to both hM4Di subjects and poor avoiders (# signifies p < 0.05); hM3Dq subjects also produced more avoidance responses relative to both hM4Di subjects and poor avoiders (* signifies p < 0.01). D Shuttles averaged across all 10 trials at test (x-axis divided into 15-sec epochs): CS presentation elevated two-way shuttling in hM3Dq subjects relative to their pre-CS baseline (# signifies p < 0.05), and this trend persisted into the first two epochs of the inter-trial interval (ITI) (* signifies p < 0.01); CS presentation also elevated shuttling in GFP controls, and this trend persisted into the first epoch of the ITI only (# signifies p < 0.05). E Locomotor activity (centimeters traveled) averaged across trials at test: CS presentation increased locomotion in hM3Dq and GFP subjects relative to poor avoiders only, and this trend persisted into the first epoch of the inter-trial interval (ITI) (# signifies p < 0.05); locomotor activity was elevated in the hM3Dq group relative to both the hM4Di group and the poor avoiders during the 4th epoch of the ITI and relative to the poor avoiders only during the 5th epoch of the ITI (# signifies p < 0.05). F Average freezing during the CS: poor avoiders froze more than GFP and hM3Dq subjects (* signifies p < 0.01); hM4Di subjects froze more than hM3Dq subjects (# signifies p < 0.05). G Freezing averaged across trials at test: CS presentation increased freezing in poor avoiders relative to their pre-CS baseline, and this trend continued into the first epoch of the ITI (# signifies p < 0.05). H Correlation of avoidance responses per CS and average freezing during the CS for all subjects (r = –0.87, p < 0.01). Other than the correlation scatter plot, all graphs depict mean (±SEM).
All groups showed normal avoidance during training, with the exception of poor avoiders (Fig. 5B). We first examined the effect of our chemogenetic manipulations on the expression of the avoidance response at test (Fig. 5C). One-way ANOVA conducted on total avoidance responses revealed a main effect for group [F(3,27)=11.30, p = 0.00005]. Fisher’s LSD post-hoc tests further revealed that this effect was driven by a significant decrease in avoidance responses in both the hM4Di and poor avoider groups relative to the GFP group (p = 0.010 and p = 0.0014, respectively). Fisher’s LSD post-hoc tests also demonstrated a significantly elevated response level in the hM3Dq group relative to the hM4Di and poor avoider groups (p = 0.00015 and p = 0.00003, respectively), but not GFP controls. These data replicate and extend the above finding that BNST inhibition attenuated the expression of the avoidance response.
To perform a more granular analysis of responses during test, we divided each trial into nine 15 s epochs, starting with a pre-CS baseline period and continuing through the CS and inter-trial interval (ITI). The number of two-way shuttles (transitions from one side of the chamber to the other) occurring in each epoch was averaged across trials to create a representative picture of behavior across the test session (Fig. 5D). These data were subjected to a two-way, mixed-design ANOVA with a between-subjects factor of Group (GFP, hM4Di, hM3Dq, or poor avoider) and a within-subjects factor of Time (epoch), which revealed a significant Group X Time interaction [F(24,216) = 7.4939, p < 0.00001]. To interrogate the source of this effect, we used a Dunnett’s multiple comparisons test to compare the average number of shuttles during each group’s pre-CS baseline to every subsequent epoch. This analysis revealed that CS presentation significantly elevated two-way shuttling relative to the pre-CS baseline in both hM3Dq (p = 0.016) and GFP (p = 0.012) groups. Interestingly, hM3Dq subjects showed increased shuttling through the first and second post-CS epochs of the ITI (p = 0.00031 and p = 0.0048, respectively) before falling back to pre-CS baseline levels. In contrast, ITI shuttling in the GFP group remained elevated above the pre-CS baseline during the first post-CS epoch only (p = 0.0197). Notably, neither hM4Di nor poor avoider groups showed any significant deviation from their pre-CS baseline levels of two-way shuttling. Thus, the CS increased the expression of shuttling in animals receiving chemogenetic (hM3Dq) activation of BNST as well as in GFP controls, and though this effect endured beyond the CS to some extent in both groups, it did so for longer in the case of BNST activation. In contrast, chemogenetic (hM4Di) inhibition of BNST eliminated the effects of the CS on expression of two-way shuttling, producing a pattern of behavior highly similar to poor avoiders, despite the fact the hM4Di subjects showed robust avoidance during training.
Next, we analyzed locomotor activity (distance traveled in cm) averaged across the same time bins as above (Fig. 5E). This allowed us to assess whether changes in shuttling might be due to general enhancements or impairments in locomotion caused by our manipulations of BNST. Locomotor activity data were subjected to a two-way, mixed-design ANOVA with a between-subjects factor of Group and a within-subjects factor of Time, as above. This analysis revealed a significant Group X Time interaction [F(24,208) = 3.115, p < 0.00001], which we parsed using a Dunnett’s multiple comparisons test in which distance traveled during each group’s pre-CS baseline was contrasted with every subsequent time bin, identical to the post-hoc analysis described above. Intriguingly, this analysis uncovered no statistically significant deviations from the pre-CS baseline in any group. To uncover the source of the interaction revealed by the ANOVA, we then conducted a Tukey’s multiple comparisons test to examine how locomotor activity differed between groups within each epoch. Notably, there were no statistically significant differences between any group during the pre-CS baseline. During the CS, significant differences in distance traveled were observed between poor avoiders and the hM3Dq and GFP groups (p = 0.013 and p = 0.026, respectively). The difference between poor avoiders and hM3Dq subjects was also present in the 1st and 2nd ITI epochs (p = 0.0089 and p = 0.043, respectively). In contrast, the difference between poor avoiders and GFP controls and was only significant in the 1st ITI epoch following the CS (p = 0.0099). In addition, significant differences between poor avoider and hM3Dq groups remerged during the 4th and 5th ITI following the CS (p = 0.032 and p = 0.036, respectively). The only significant difference that did not involve the poor avoiders was between hM3Dq and hM4Di groups during the 4th ITI epoch after the CS (p = 0.032). Thus, the differences revealed in this analysis seem to be driven by reduced locomotion in poor avoiders more than any other factor. We conclude that the effects of BNST activation and inhibition on shuttling cannot be attributed to alterations in locomotion.
We also measured CS-evoked freezing at test (Fig. 5F). Time spent freezing was averaged over ten CSs for each subject and then analyzed with a one-way ANOVA that revealed a significant main effect for Group [F(3,27) = 4.917, p = 0.0075]. Fisher’s LSD post-hoc tests further revealed that the hM4Di group froze more than the hM3Dq group (p = 0.0199), though freezing in hM4Di subjects was not significantly different than GFP controls or poor avoiders. In addition, Fisher’s LSD post-hoc tests revealed that poor avoiders froze significantly more than both hM3Dq subjects (p = 0.0018) and GFP controls (p = 0.011), consistent with the high level of freezing previously observed in subjects that fail to express the avoidance response [10, 14]. Neither chemogenetic activation nor inhibition altered freezing during the CS relative to GFP controls, suggesting that changes in the avoidance response caused by these manipulations cannot be accounted for by changes in freezing.
Next, we again broke each trial into 15-sec epochs starting with a pre-CS baseline and averaging the time spent freezing during each consecutive epoch across trials (Fig. 5G). These data were analyzed using a two-way, mixed-design ANOVA with a between-subjects factor of Group (GFP, hM4Di, hM3Dq, and poor avoider) and a within-subjects factor of Time (epoch), which revealed a significant Group X Time interaction [F(24,216) = 4.127, p < 0.00001]. We then interrogated this effect using a Dunnett’s multiple comparisons test to compare time spent freezing during each group’s pre-CS baseline to freezing in all subsequent epochs. The only significant differences that this analysis revealed were in the poor avoider group, which froze more relative to the pre-CS baseline during both the CS and the 1st epoch of the ITI following the CS (p = 0.043 and 0.048, respectively). Freezing did not differ significantly from pre-CS baseline in any other group. The overall flatness of freezing across the trial may be due to the fact that the training environment acquired an aversive association strong enough to produce substantial levels of contextual freezing. However, this relatively invariant pattern of trial-wide freezing did not interfere with group-level differences in shuttling or avoidance.
Because prior research shows that manipulations which attenuate avoidance also tend to produce concurrent increases in CS-evoked freezing [15, 27], we analyzed the correlation between avoidance responses/CS and freezing/CS in all subjects (Fig. 5H). Pearson’s r-coefficient analysis revealed a strong negative correlation between the two behaviors [r = –0.8657, p < 0.00001]. Thus, at the level of individual subjects, there was an inverse correlation between freezing and avoidance, even though group-level differences in freezing did not result from our chemogenetic manipulations.
Overall, these results confirm that inhibition of BNST impairs performance of the avoidance response, and extend that result by revealing that activation of BNST can produce a pattern of elevated responses that persists beyond the CS.
Discussion
The data presented here are the first demonstration that the bed nucleus of the stria terminalis (BNST) is both necessary for the expression of two-way signaled active avoidance (SAA) and sufficient to enhance the output of this response in male rats. Other regions that contribute to avoidance in males include the basolateral complex of the amygdala [10] and the infralimbic cortex [15], both of which send excitatory projections to BNST [28, 29]. BNST sends direct inputs to the nucleus accumbens shell [28], which has also been implicated in the expression of two-way avoidance [27], as well as to the ventral tegmental area [30], which is the source of nucleus accumbens dopamine [31]. Thus, BNST is situated to act as a key hub in a broader system for proactive defensive behavior in male rats.
Our data also suggest that BNST is not necessary for two-way avoidance in females. There are a number of ways to interpret this sex difference. One possibility is that a key variation in cell physiology makes neural activity in the BNST of female rats less susceptible to chemogenetic inhibition. Though we cannot completely rule out this possibility, we discount it because BNST inhibition reduced shuttles during the acclimation period in both males and females, suggesting that the inhibitory hM4Di DREADD is operative in BNST neurons in both sexes. Another possibility is that BNST does indeed underpin avoidance in females, but at a different, perhaps earlier training timepoint than that tested here. If so, it is likely to make a relatively brief contribution, dropping out before the subject reaches asymptotic levels of avoidance. A third possibility is that abundant sex differences in the neurochemistry and structure of the BNST [32–36] translate into sex differences in the behavioral function of this region. Thus, chemogenetic manipulations of BNST in males may target a crucial process underlying the expression of avoidance that is nested in an anatomically distinct neural substrate in females. For instance, BNST inactivation in males may suppress avoidance by preventing the vigorous expression of instrumental action, which is BNST-dependent in male mice [37]. This interpretation raises the intriguing question of whether females avoid by relying on circuits for an associative process comparable to that engaged in males, but located elsewhere in the brain, or whether females engage a different neural substrate underlying a dissociable form of learning and memory to express this behavior. Future research will explore the degree to which males and females take distinct neural and/or psychological paths to the avoidance response.
Previous work on the role of BNST in other aversive associative learning paradigms has focused on its contribution to respondent or reactive behaviors, most commonly freezing (in male rats [18, 19, 38–42], female rats [42, 43], and male mice [44, 45]), but also conditioned suppression (in female rats [46]), fear-potentiated startle (in male rats [47]), and flight (in male and female rats [48]). Evidence presented here, demonstrating that BNST supports a proactive aversive response in males, is a novel addition to the broadening repertoire of defensive behaviors underpinned by this region. The functional heterogeneity of BNST is reflected in its anatomical complexity. BNST is a collection of small, interconnected sub-nuclei comprised of multiple cell types [49–52]. Distinct BNST populations have been shown to underpin specific defensive responses [52–57], suggesting that contrasting behavioral functions may indeed be supported by dissociable BNST pathways, similar to what meticulous circuit-dissection work has revealed for a related structure, the central amygdala [58–60].
In addition to experiments that targeted BNST, we also performed a chemogenetic inactivation of the medial septum (MS) in male rats and found no effect of this manipulation on the two-way avoidance response. However, previous studies have used a permanent lesion methodology to demonstrate a modulatory role for MS in SAA [61, 62]. One such study, using a lower shock intensity (0.5 mA) than the 0.7 mA US employed here, demonstrated that MS lesion in male rats enhanced the expression of avoidance [62]. Another study, also in males, showed that MS lesion accelerated acquisition of avoidance at an even lower shock intensity (0.4 mA), though no effect for acquisition was observed for the 0.7 mA shock we used [61]. The authors interpreted these results as an effect on exploratory behavior related to a non-associative process, such as habituation [62]. Given that these faciliatory effects were restricted to lower-magnitude USs, they are consistent with the non-effect of MS inactivation at the higher US magnitude described here.
Recent empirical [63] and theoretical [64, 65] reports argue that two-way avoidance should be categorized as a pre-encounter defensive behavior within the framework of threat imminence theory, an important conceptual tool for understanding conditioned aversion from the perspective of predator/prey dynamics in natural settings [6]. A threat imminence approach to SAA draws on ecological models of predatory defense that place proactive/preventative avoidance measures at a time point in predator/prey interactions before the predator has been directly encountered, when the threat of attack is relatively distant [7, 8, 66]. Characterizing two-way avoidance as a component of the pre-encounter defensive mode fits comfortably with an important theme of previous research on BNST, which is that BNST-dependent conditioned defensive responses tend to be evoked by threats that are distal, ambiguous, or otherwise difficult to predict (in both males [18, 19, 27] and females [42]). As a substrate of distal threat processing, the BNST may be recruited to SAA by the increasing psychological distance of the US that occurs as the rising frequency of the avoidance response decreases the incidence of shock, transforming a threat that is certain during early trials into one that is merely possible once the subject achieves stable levels of avoidance expression. The fact that an identical series of events does not recruit the BNST in females suggests either that the relationship between uncertain threat and proactive responses is mediated by a distinct substrate or that a distinct form of threat processing underpins two-way avoidance in female rats.
The pre-encounter defensive functions supported by BNST are relevant to anxious pathology in humans, which is characterized by a disruptive, adverse response to nebulous or far-off threats [67–69]. Though avoidance can function as a normal defensive behavior [3], excessive avoidance is a common behavioral symptom of multiple forms of clinical anxiety [1] that can prolong the underlying anxious pathology by buffering it against therapeutic intervention [70, 71]. Our data connect BNST to avoidant behavior in males but not females, revealing the complexity of the circuits underlying pre-encounter defense, an important and underexplored area of aversive behavior. Ongoing research will examine the relationship between threat imminence, avoidance, sex, and the function of BNST, in order to create a fuller understanding of threat-processing mechanisms and defensive behaviors highly relevant to anxiety disorder.
Acknowledgements
We thank Dr. Jun Wang for providing the CNO for our slice physiology experiment.
Author contributions
DPG assisted in the design of the behavioral experiments, executed those experiments, assisted in the analysis of behavioral data, performed the relevant histology, and contributed to the writing manuscript. WW and KS designed and executed the physiological experiment and edited the manuscript. JM designed the behavioral experiments and supervised their execution, analyzed behavioral data, and wrote the manuscript.
Funding
This work was supported by NIH grant R21MH126327 (JMM) and BBRF NARSAD48 Young Investigator Award 25196 (JMM). The authors have no competing financial interests to disclose.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (5th ed). Arlington, VA.
- 2.LeDoux JE, Moscarello JM, Sears R, Campese V. The birth, death and resurrection of avoidance: a reconceptualization of a troubled paradigm. Mol Psychiatry. 2017;22:24–36. doi: 10.1038/mp.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moscarello JM, Hartley CA. Agency and the calibration of motivated behavior. Trends Cogn Sci. 2017;21:725–35. doi: 10.1016/j.tics.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Kingston J, Clarke S, Remington B. Experiential avoidance and problem behavior: a mediational analysis. Behav Modif. 2010;34:145–63. doi: 10.1177/0145445510362575. [DOI] [PubMed] [Google Scholar]
- 5.Nesse RM. Natural selection and the regulation of defenses. Evolut Hum Behav. 2005;26:88–105. doi: 10.1016/j.evolhumbehav.2004.08.002. [DOI] [Google Scholar]
- 6.Fanselow MS, Lester LS (1988). A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In R. C. Bolles & M. D. Beecher (Eds.), Evolution and learning (pp. 185–212). Lawrence Erlbaum Associates, Inc.
- 7.Mobbs D, Headley DB, Ding W, Dayan P. Space, time, and fear: survival computations along defensive circuits. Trends Cogn Sci. 2020;24:228–41. doi: 10.1016/j.tics.2019.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Mobbs D, Kim JJ. Neuroethological studies of fear, anxiety, and risky decision-making in rodent and humans. Curr Opin Behav Sci. 2015;5:8–15. doi: 10.1016/j.cobeha.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perusini JN, Fanselow MS. Neurobehavioral perspectives on the distinction between fear and anxiety. Learn Mem. 2015;22:417–25. doi: 10.1101/lm.039180.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi JS, Cain CK, LeDoux JE. The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learn Mem. 2010;17:139–47. doi: 10.1101/lm.1676610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamin LJ, Brimer CJ, Black AH. Conditioned suppression as a monitor of fear of the CS in the course of avoidance training. J Comp Physiol Psychol. 1963;56:497–501. doi: 10.1037/h0047966. [DOI] [PubMed] [Google Scholar]
- 12.LaPointe T, Worr M, Leri F. Analysis of memory modulation by conditioned stimuli. Learn Mem. 2021;28:87–94. doi: 10.1101/lm.052407.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mineka S, Gino A. Dissociation between conditioned emotional response and extended avoidance performance. Learn Motiv. 1980;11:476–502. doi: 10.1016/0023-9690(80)90029-6. [DOI] [Google Scholar]
- 14.Moscarello JM. Prefrontal cortex projections to the nucleus reuniens suppress freezing following two-way signaled avoidance training. Learn Mem. 2020;27:119–23. doi: 10.1101/lm.050377.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moscarello JM, LeDoux JE. Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. J Neurosci. 2013;33:3815–23. doi: 10.1523/JNEUROSCI.2596-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clauss JA, Avery SN, Benningfield MM, Blackford JU. Social anxiety is associated with BNST response to unpredictability. Depress Anxiety. 2019;36:666–75. doi: 10.1002/da.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–35. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goode TD, Acca GM, Maren S. Threat imminence dictates the role of the bed nucleus of the stria terminalis in contextual fear. Neurobiol Learn Mem. 2020;167:107116. doi: 10.1016/j.nlm.2019.107116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goode TD, Ressler RL, Acca GM, Miles OW, Maren S. Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals. eLife. 2019;8:e46525. doi: 10.7554/eLife.46525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armbuster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci. 2007;104:5163–8. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lázaro-Muñoz G, LeDoux JE, Cain CK. Sidman instrumental avoidance depends on lateral and basal amygdala and is constrained by central amygdala-mediated pavlovian processes. Biol Psychiatry. 2010;67:1120–7. doi: 10.1016/j.biopsych.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swanson LW (2004) Brain maps: structure of the rat brain, 3rd edition.
- 24.Rodriguez-Sierra OE, Turesson HK, Paré D. Contrasting distribution of physiological cell types in different regions of the bed nucleus of the stria terminalis. J Neurophysiol. 2013;110:2017–49. doi: 10.1152/jn.00408.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzone CM, Pati D, Michaelides M, DiBerto J, Fox JH, Tipton G, et al. Acute engagement of Gq-mediated signaling in the bed nucleus of the stria terminalis induces anxiety-like behavior. Mol Psychiatry. 2018;23:143–53. doi: 10.1038/mp.2016.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gungor NZ, Paré Functional heterogeneity in the bed nucleus of the stria terminalis. J Neurosci. 2016;36:8038–49. doi: 10.1523/JNEUROSCI.0856-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez F, Moscarello JM, LeDoux JE, Sears RM. Active avoidance requires a serial basal amygdala to nucleus accumbens circuit. J Neurosci. 2015;35:34703477. doi: 10.1523/JNEUROSCI.1331-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glover LR, McFadden KM, Bjorni M, Smith SR, Rovero NG, Oreizi-Esfahani S, et al. A prefrontal-bed nucleus of the stria terminalis circuit limits fear to uncertain threat. eLife. 2020;9:e60812. doi: 10.7554/eLife.60812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dabrowska J, Martinon D, Moaddab M, Rainnie DG (2016) Targeting corticotropin-releasing factor projections from the oval nucleus of the bed nucleus of the stria terminalis using cell-type specific neuronal tracing studies in mouse and rat brain. J Neuroendocrinol. 28: 10.1111/jne12442. [DOI] [PMC free article] [PubMed]
- 30.Shackman AJ, Fox AS. Contributions of the central extended amygdala to fear and anxiety. J Neurosci. 2016;36:8050–63. doi: 10.1523/JNEUROSCI.0982-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yetnikoff L, Lavezzi HN, Reichard RA, Zahm DS. An update of the connections of the ventral mesencephalic dopaminergic complex. Neuroscience. 2015;282:23–48. doi: 10.1016/j.neuroscience.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.del Abril A, Segovia S, Guillamón A. The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Dev Brain Res. 1987;32:295–300. doi: 10.1016/0165-3806(87)90110-6. [DOI] [PubMed] [Google Scholar]
- 33.Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579:321–6. doi: 10.1016/0006-8993(92)90068-K. [DOI] [PubMed] [Google Scholar]
- 34.Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–9. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Tsukahara S, Morishita M. Sexually dimorphic formation of the preoptic area and the bed nucleus of the stria terminalis by neuroestrogens. Front Neurosci. 2020;14:797. doi: 10.3389/fnins.2020.00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uchida K, Otsuka H, Morishita M, Tsukahara S, Sato T, Sakimura K, et al. Female-biased sexual dimorphism of corticotropin-releasing factor neurons in the bed nucleus of the stria terminalis. Biol Sex Differ. 2019;10:6. doi: 10.1186/s13293-019-0221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge M, Balleine BW. The role of the bed nucleus of the stria terminalis in the motivational control of instrumental action. Front Behav Neurosci. 2022;16:968593. doi: 10.3389/fnbeh.2022.968593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjorni M, Rovero NG, Yang ER, Holmes A, Halladay LR. Phasic signaling in the bed nucleus of the stria terminalis during fear learning predicts within- and across-session cued fear expression. Learn Mem. 2020;27:83–90. doi: 10.1101/lm.050807.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hott SC, Gomes FV, Uliana DL, Vale GT, Tirapelli CR, Resstel LBM. Bed nucleus of the stria terminalis NMDA receptors and nitric oxide modulate contextual fear conditioning in rats. Neuropharmacology. 2017;112(part A):135–43. doi: 10.1016/j.neuropharm.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, LeDoux JE. Lesions of the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Zimmerman JM, Maren S. The bed nucleus of the stria terminalis is required for the expression of contextual but not auditory freezing in rats with basolateral amygdala lesions. Neurobiol Learn Mem. 2011;95:199–205. doi: 10.1016/j.nlm.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urien L, Stein N, Ryckman A, Bell L, Bauer EP. Extended amygdala circuits are differentially activated by context fear conditioning in male and female rats. Neurobiol Learn Mem. 2021;180:107401. doi: 10.1016/j.nlm.2021.107401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammack SE, Todd TP, Kocho-Schellenberg M, Bouton ME. Role of the bed nucleus of the stria terminalis in the acquisition of contextual fear at long or short context-shock intervals. Behav Neurosci. 2015;129:673–8. doi: 10.1037/bne0000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asok A, Draper A, Hoffman AF, Schulkin J, Lupicia CR, Rosen JB. Optogenetic silencing of a corticotropin-releasing factor pathway from the central amygdala to the bed nucleus of the stria terminalis disrupts sustained fear. Mol Psychiatry. 2018;23:914–22. doi: 10.1038/mp.2017.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruzsik B, Biro L, Zelena D, Sipos E, Szebik H, Sarosdi KR, et al. Somatostatin neurons of the bed nucleus of stria terminalis enhance associative fear memory in mice. J Neurosci. 2021;41:1982–95. doi: 10.1523/JNEUROSCI.1944-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and the reinstatement of extinguished fear. Behav Neurosci. 2006;120:324–36. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- 47.Davis M, Walker DL. Role of the bed nucleus of the stria terminalis and amygdala AMPA receptors in the development and expression of context conditioning and sensitization of startle by prior shock. Brain Struct Funct. 2014;219:1969–82. doi: 10.1007/s00429-013-0616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Totty MS, Warren N, Huddleston I, Ramanathan KR, Ressler RL, Oleksiak CR, et al. Behavioral and brain mechanisms mediating conditioned flight behavior in rats. Sci Rep. 2021;11:8215. doi: 10.1038/s41598-021-87559-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Avery SN, Clauss JA, Blackford JU. The human BNST: functional role in anxiety and addiction. Neuropsychopharamcology. 2016;41:126–41. doi: 10.1038/npp.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LeBow MA, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 2016;21:450–63. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turresson HK, Rodríguez-Sierra OE, Pare D. Intrinsic connections in the anterior part of the bed nucleus of the stria terminalis. J Neurophysiol. 2013;109:2438–50. doi: 10.1152/jn.00004.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruzsik B, Biro L, Sarosdi KR, Zelena D, Sipos E, Szebik H, et al. Neurochemically distinct populations of the bed nucleus of stria terminalis modulate innate fear response to weak threat evoked by predator odor stimuli. Neurobiol Stress. 2021;15:100415. doi: 10.1016/j.ynstr.2021.100415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crowley NA, Bloodgood DW, Hardaway A, Kendra AM, McCall JG, Al-Hasani R, et al. Dynorphin controls the gain of an amygdalar anxiety circuit. Cell Rep. 2016;14:2774–83. doi: 10.1016/j.celrep.2016.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giardino WJ, Eban-Rothschild A, Christoffel DJ, Li S-B, Malenka RC, de Lecea L. Parallel circuits from the bed nucleus of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nature. 2018;21:1084–95. doi: 10.1038/s41593-018-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–8. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim S-Y, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, et al. Diverging neural pathways assemble a behavioural state from separable features of anxiety. Nature. 2013;496:219–23. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luskin AT, Bhatti DL, Mulvey B, Pederson CE, Girven KS, Oden-Brunson H, et al. Extended amygdala-parabrachial circuits alter threat assessment and regulate feeding. Sci Adv. 2021;7:eabd3666. doi: 10.1126/sciadv.abd3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ciocchi S, Herry C, Grenier F, Wolff JJ, Vlachos I, Ehrlich I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–82. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 59.Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature. 2017;542:96–100. doi: 10.1038/nature21047. [DOI] [PubMed] [Google Scholar]
- 60.Gozzi A, Jain A, Giovannelli A, Bertollini C, Crestan V, Schwarz AJ, et al. A neural switch for active and passive fear. Neuron. 2010;67:656–66. doi: 10.1016/j.neuron.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Sagvolden T, Johnsrud G. Two-way active avoidance learning following medial, dorsolateral, or total septal lesions in rats: effects of intensity of discontinuous shock. Behav Neural Biol. 1982;35:17–32. doi: 10.1016/S0163-1047(82)91240-7. [DOI] [PubMed] [Google Scholar]
- 62.Torras-Garcia M, Costa-Miserachs D, Morgado-Bernal I, Portell-Cortés I. Improvement of shuttle-box performance by anterodorsal septal lesions in rats. Behav Brain Res. 2003;141:147–58. doi: 10.1016/S0166-4328(02)00346-7. [DOI] [PubMed] [Google Scholar]
- 63.Laughlin LC, Moloney DM, Samels SB, Sears RM, Cain CK. Reducing shock imminence eliminates poor avoidance in rats. Learn Mem. 2020;27:270–4. doi: 10.1101/lm.051557.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cain CK. Avoidance problems reconsidered. Curr Opin Behav Sci. 2019;26:9–17. doi: 10.1016/j.cobeha.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moscarello JM, Penzo MA. The central nucleus of the amygdala and the construction of defensive modes across the threat-imminence continuum. Nat Neurosci. 2022;25:999–1008. doi: 10.1038/s41593-022-01130-5. [DOI] [PubMed] [Google Scholar]
- 66.Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool. 1990;68:619–40. doi: 10.1139/z90-092. [DOI] [Google Scholar]
- 67.Knight LK, Depue BE. New frontiers in anxiety research: the translational potential of the bed nucleus of the stria terminalis. Front Psychiatry. 2019;10:510. doi: 10.3389/fpsyt.2019.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LeBow MA, Chen A. Overshadowed by the amygdala: the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 2016;21:450–63. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miles OW, Maren S. Role of the bed nucleus of the stria terminalis in PTSD: insights from preclinical models. Front Behav Neurosci. 2019;13:68. doi: 10.3389/fnbeh.2019.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blakey SM, Abramowitz JS. The effects of safety behaviors during exposure therapy for anxiety: Critical analysis from an inhibitory learning perspective. Clin Psychol Rev. 2016;49:1–15. doi: 10.1016/j.cpr.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Meacham F, Bergstrom C. Adaptive behavior can produce maladaptive anxiety due to individual differences in experience. Evol Med Public Health. 2016;2016:270–85. doi: 10.1093/emph/eow024. [DOI] [PMC free article] [PubMed] [Google Scholar]