Abstract
Background
Transcatheter aortic valve replacement (TAVR) has become the dominant treatment strategy for severe aortic stenosis in patients with high and intermediate surgical risk. Although complications are significant cause of increasing mortality after TAVR and bailout techniques have been well established, we still encounter a rare complication without widely accepted bailout option. We present a rare complication of valvuloplasty balloon entrapment to a self-expanding valve strut with successful bailout.
Case summary
A 71-year-old man complaining of dyspnoea underwent valve-in-valve TAVR for failed surgical aortic valve. However, he developed acute decompensated heart failure due to high residual aortic gradient (peak aortic velocity of 4.0 m/s and mean aortic gradient of 37 mmHg) on the 3rd day after TAVR. Computed tomography demonstrated underexpansion of transcatheter heart valve (THV) within the surgical valve. Therefore, urgent balloon valvuloplasty was performed. The balloon entrapment in the THV stent frame happened during the procedure. Percutaneous removal through transseptal approach using snaring technique was successfully performed.
Discussion
Balloon entrapment within a THV is a rare complication and potentially requires urgent surgical removal. To our knowledge, this is the first report utilizing the snaring technique through transseptal approach for balloon entrapment within a THV. Through the current report, we highlight the utility and effectiveness of the transseptal snaring technique with using a steerable transseptal sheath. Moreover, this case shows the importance of the multiprofessional approach to resolve unexpected complications.
Keywords: Case report, Transcatheter aortic valve replacement, Complication, Valvuloplasty balloon entrapment, Transseptal snaring technique
Learning points.
Transseptal snaring technique is an option for removing entrapped valvuloplasty balloon in a transcatheter heart valve (THV) stent frame.
Valvuloplasty balloon can be entrapped within a THV, especially a self-expanding valve. It is necessary to confirm the wire passage carefully with multi-angle images.
Introduction
Transcatheter aortic valve replacement (TAVR) is a well-established treatment for patients with severe aortic stenosis along with surgical aortic valve replacement (SAVR).1,2 Bailout strategies for TAVR-related complications such as atrio-ventricular conduction disturbances, coronary artery and vascular injuries, and valve malpositioning have been reported.3,4 We recently encountered a rare complication for which widely accepted treatment option has not been described. In our case, the aortic valvuloplasty balloon, which was entrapped in the self-expanding aortic valve stent frame, was successfully removed via femoral vein using transseptal snaring technique.
Timeline
| Time | Events |
| 6 years earlier | Surgical aortic valve replacement (SAVR) |
| 5 years earlier | Re-do SAVR with a 23 mm Trifecta bioprosthesis due to endocarditis |
| 6 months earlier | Patient with dyspnoea being diagnosed as structural valve deterioration (peak velocity of 4.1 m/s, mean gradient of 40 mmHg, and no paravalvular leak) |
| Day 0 | Valve-in-valve transcatheter aortic valve replacement (Evolut PRO + 26-mm) |
| Day 1 | Transthoracic echocardiography with high residual aortic gradient (peak aortic velocity of 4.0 m/s, mean aortic gradient of 37 mmHg) and computed tomography showing underexpansion of transcatheter heart valve |
| Day 3 | Development of acute decompensated heart failure |
| Day 5 | Balloon aortic valvuloplasty (Atlas Gold PTA Balloon Dilatation Catheter 20-mm), leading to balloon entrapment to the strut of Evolut PRO+. Subsequent successful removal of entrapped balloon with transseptal snaring technique. |
| Day 7 | Transthoracic echocardiography showing decrease of aortic gradient (peak aortic velocity of 2.0 m/s, mean aortic gradient of 8 mmHg) |
| Day 13 | Discharge to home without any symptoms |
Case presentation
A 71-year-old man with heart failure [New York Heart Association (NYHA) functional Class II] suffered from severe aortic stenosis due to surgical aortic valve failure. He had undergone SAVR 6 years earlier, and re-do SAVR due to endocarditis had been performed with a 23 mm Trifecta bioprosthesis (Abbott, Minneapolis, MN, USA) (true internal diameter of 21 mm) 5 years earlier. Computed tomography (CT) before re-do SAVR had shown a pseudoaneurysm at sinus of valsalva just below the ostium of left coronary artery (see Supplementary material online, Figure S1A) and a large vegetation extending from the surgical bioprosthetic valve to the ostium of right coronary artery (see Supplementary material online, Figure S1B). In the reoperation, coronary artery bypass grafting (saphenous vein graft to right coronary artery) had been performed first because the vegetation had already provoked non-ST-segment elevation myocardial infarction. Afterwards, the vegetation and the surgical bioprosthetic valve had been removed. Removing the valve had caused an iatrogenic ventricular septal defect (VSD) at membranous septum below non-coronary cusp. The pseudoaneurysm and the VSD had been closed with two separate bovine pericardial patches (see Supplementary material online, Figure S1C). Finally, a 23 mm Trifecta bioprosthesis had been implanted. Transoesophageal echocardiography (TEE) immediately after re-do SAVR had demonstrated good mobility of all leaflets. On the ninth postoperative day, transthoracic echocardiography (TTE) had shown minor residual aortic pressure gradient (peak aortic velocity of 2.1 m/s and mean aortic gradient of 9 mmHg).
He had been diagnosed with stenosis of Trifecta bioprosthesis with good left ventricular function 54 months after re-do SAVR (peak aortic velocity of 4.1 m/s, mean aortic gradient of 40 mmHg, no paravalvular leak, and left ventricular ejection fraction of 65%), and he was referred to our hospital. Physical examination revealed systolic heart murmur, normal respiratory sounds in auscultation, and no leg oedema. Routine laboratory values were normal.
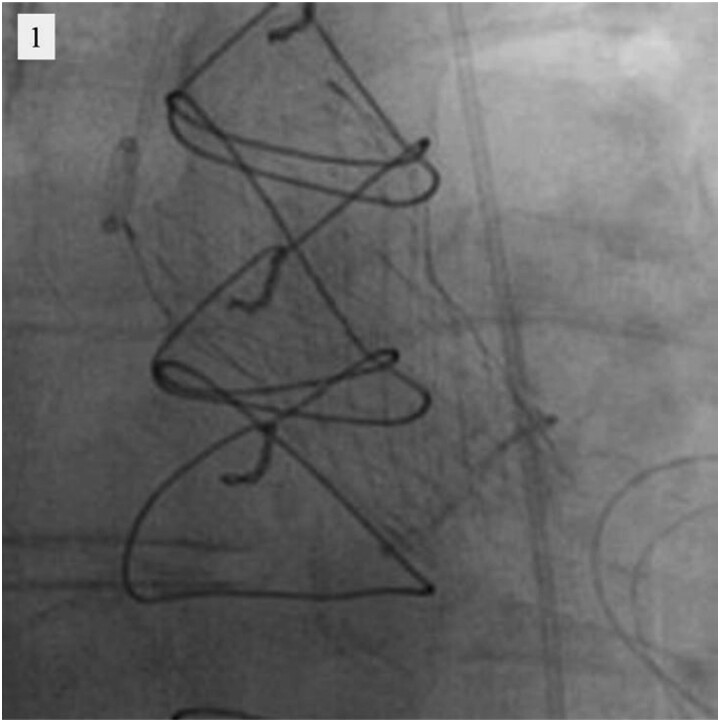
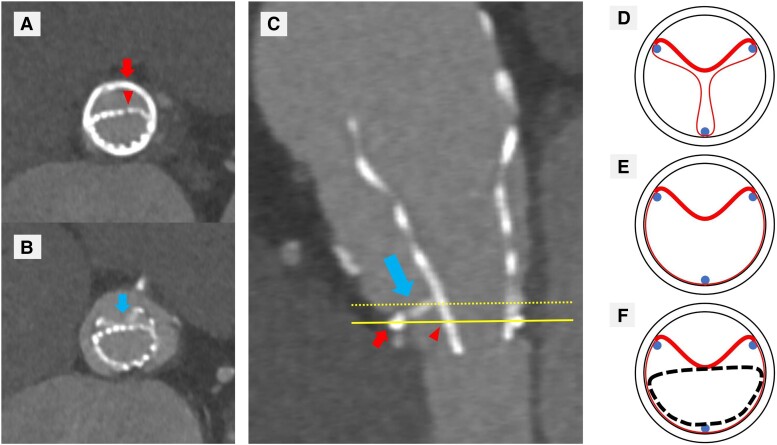
Taking the history of two prior open-heart operations into consideration, our heart team decided to perform valve-in-valve TAVR. In the procedure, a 26 mm Evolut PRO+ (Medtronic, Minneapolis, MN, USA) transcatheter heart valve (THV) was deployed at proper position within the surgically implanted bioprosthetic valve using transfemoral approach (Figure 1). Intraprocedural transvalvular gradients were not measured because there were no findings of THV underexpansion in the implant view on fluoroscopy and his vital signs were stable. However, on the first postoperative day, TTE showed high residual gradient in the THV (peak aortic velocity of 4.0 m/s and mean aortic gradient of 37 mmHg), and subsequent CT revealed pronounced underexpansion of the valve (Figure 2A). Considering CT findings, the immobile right coronary leaflet of the surgical bioprosthesis due to severe calcification caused the underexpansion of the THV (Figure 2B and C). It was considered that this immobile leaflet had provoked stenosis of the surgical bioprosthesis before valve-in-valve TAVR (Figure 2D and E), and the leaflet was not expanded even after the procedure (Figure 2F). Two days later, it was decided to perform urgent balloon aortic valvuloplasty (BAV) because the patient had severe congestive heart failure and pulmonary oedema requiring mechanical ventilation.
Figure 1.
Valve-in-valve transcatheter aortic valve replacement fluoroscopy.
Figure 2.
Computed tomography (A–C) showing underexpansion of transcatheter heart valve and schematic diagrams explaining this phenomenon (D–F). (A–C) The small arrows show sewing ring of Trifecta bioprosthesis, and the arrowheads show struts of Evolut PRO+. The big arrows show the right coronary leaflet of Trifecta bioprosthesis. (A) Axial view at the level of solid line showing underexpansion of Evolut PRO+. (B) Axial view at the level of dotted line showing Evolut PRO+ compressed by the calcified immobile leaflet of Trifecta bioprosthesis. (C) Sagittal view. (D–F) The circles show sewing ring, the dots show commissural posts, the bold lines in the circles show calcified immobile leaflets, and the skinny lines in the circles show normal leaflets of Trifecta bioprosthesis. The dotted line shows struts of Evolut PRO+. (D) Schematic diagram before valve-in-valve procedure in diastole. (E) Schematic diagram before valve-in-valve procedure in systole showing a calcified immobile leaflet within struts of Trifecta bioprosthesis. (F) Schematic diagram after valve-in-valve procedure in systole showing struts of Evolut PRO+ compressed by the calcified immobile leaflet of Trifecta bioprosthesis.
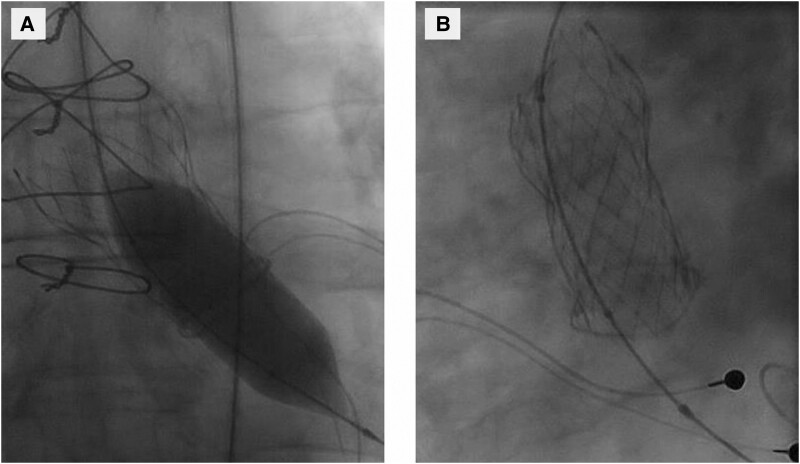
BAV was performed via right femoral artery. The THV was initially crossed with a 5 Fr Amplatz left coronary catheter and 0.035 × 300 cm J-curved wire under fluoroscopic control. Next, the Amplatz catheter was removed, and a 5 Fr pigtail was introduced into left ventricle over the wire. The wire was replaced with a 0.035 × 260 cm Amplatz Extra Stiff Wire, and BAV was performed with a 20 × 40 mm Atlas Gold PTA Balloon Dilatation Catheter (Bard Peripheral Vascular, Tempe, AZ, USA) (Figure 3A). However, when removing the equipment, it was noticed that the balloon was entrapped within the THV (Figure 3B). It turned out that the wire and the balloon had passed through the frame of the THV. It was possible to push the balloon further in to the left ventricle, but all attempts to pull it out via aorta failed.
Figure 3.
Balloon aortic valvuloplasty fluoroscopy. (A) Dilation of the transcatheter heart valve with Atlas Gold PTA Balloon Dilatation Catheter 20-mm. (B) The entrapment of balloon to an upper strut of the transcatheter heart valve.
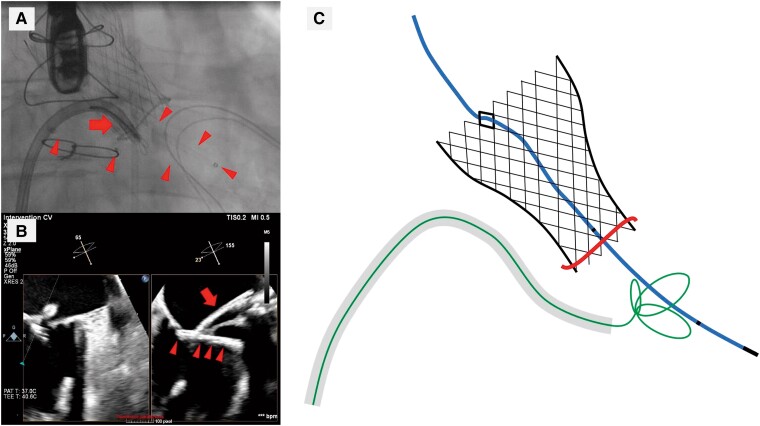
After careful evaluation of the surgical risk and other treatment options, we decided to try to remove the balloon using transseptal approach via right femoral vein. Transseptal puncture was performed using an 8.5 Fr Fast-Cath Transseptal Introducer SL1 and BRK-1 Transseptal Needle (Abbott, Minneapolis, MN, USA) under fluoroscopic and TEE guidance. Thereafter, the SL1 sheath was replaced with an 8.5 Fr Agilis NxT Steerable Introducer (Abbott, Minneapolis, MN, USA) which was advanced into the left ventricle over the guide wire. Subsequently, a 13 mm Needle’s Eye Snare (Cook Medical, Bloomington, IN, USA) was advanced into left ventricle through the steerable sheath, and the balloon was snared under fluoroscopy and TEE (Figure 4A–C). After the balloon was pulled to the tip of the transseptal sheath, the proximal shaft of the balloon catheter was cut, and the sheath and the balloon catheter were removed through the right femoral vein (see Supplementary material online, Video S1 and Supplementary material online, Video S2). The balloon was retracted in the shape folded in two at the grasped point and could be removed without disintegration (Figure 5A and B). Haemostasis after sheath removal was achieved by the Figure-of-8-Suture and compression bandage. Transoesophageal echocardiography showed small iatrogenic atrial septal defect (ASD) with minor left-to-right shunt requiring no ASD closure.
Figure 4.
Transseptal bailout for entrapment of balloon with the snare. The valvuloplasty balloon (arrowheads) being grasped via steerable transseptal sheath with a 13 mm Needle’s Eye Snare (arrows). (A) Fluoroscopy. (B) Transoesophageal echocardiography. (C) Schematic diagram during snaring the balloon.
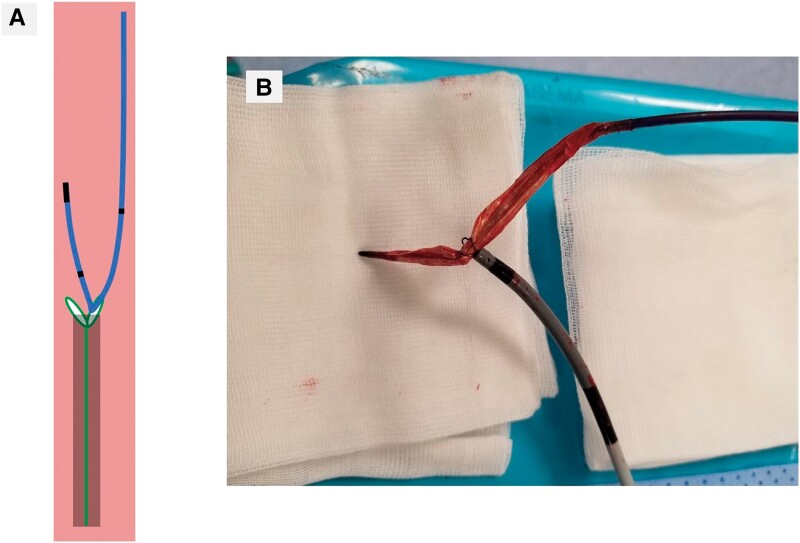
Figure 5.
The valvuloplasty balloon being grasped with the snare. (A) Schematic diagram during retracting the balloon with the snare and the transseptal sheath in a vein. (B) The valvuloplasty balloon being removed with the snare from the body.
After the BAV and successful bailout from the perioperative complication, the heart failure symptoms improved, and the patients was discharged on Day 13 with NYHA I functional status. At discharge, TTE showed minor residual aortic pressure gradient (peak aortic velocity of 2.0 m/s and mean aortic gradient of 8 mmHg).
Discussion
We report a case of successful management of aortic valvuloplasty balloon entrapment using transseptal snaring technique. Entrapment of a valvuloplasty catheter to the THV struts is a rare complication, and information on bailout strategies is limited. In percutaneous coronary intervention, several techniques for retracting entrapped balloon or intravascular ultrasound catheter have been proposed.5,6 None of these worked in the current case. We tried to mobilize the balloon by pushing, pulling, rotating, and by inflating and deflating it in the annular position, but with no success. We did not try to loosen the balloon from the THV strut by inflating it inside the strut, because it may deform or dislocate the valve or cause balloon disintegration according to prior reports. Likewise, we did not use another balloon to stabilize the THV, because the second balloon would likely interfere with the first one.7
Knowing that in-hospital mortality among TAVR patients needing open-heart surgery is as high as 46%,8 bailout surgery was considered inacceptable in this case. Instead, it was decided to use percutaneous transseptal access, snare the balloon, and pull it away through femoral vein. Transseptal puncture is commonly used in electrophysiology, and snares are routinely used in pacing lead extraction. In BAV, transseptal snaring technique at descending aorta has been used to cross aortic valve with a retrograde wire,9 but to our knowledge, transseptal snaring has not been used to manage valvuloplasty balloon entrapment. We performed transseptal puncture and snared the balloon under fluoroscopic and TEE guidance. The use of steerable transseptal sheath was extremely helpful as it allowed easy and accurate control of the needle eye snare.
Potential limitation of the transseptal approach is that the balloon and the stump of balloon catheter shaft can cause iatrogenic ASD and vascular injury while being retracted. In this case, only minor, haemodynamically insignificant ASD was observed after the procedure.10
Valvuloplasty balloon entrapment within a THV is rare. The risk is higher in self-expanding valve than in balloon-expandable valve since the former has smaller strut size and the struts are in higher position.11 In this case, the inadvertent wire passage through the THV strut was not recognized initially, because the wire, the catheter, and even the balloon moved smoothly under fluoroscopy. However, only single fluoroscopic projection was used while introducing the guide wire and the balloon catheter to left ventricle. In retrospect, it is probable that we would have been able to prevent inadvertent wire passage and valvuloplasty balloon entrapment if we had used multiple fluoroscopic views when re-crossing the THV.
In conclusion, when treating rare complications like the current one, physicians should think ‘outside the box’. Here, we worked together with an electrophysiologist having vast experience in transseptal catheterization, use of steerable transseptal sheaths, and snaring techniques. This case indicates that transseptal snaring technique is a promising bailout strategy as an alternative to open-heart surgery for aortic valvuloplasty balloon entrapment.
Supplementary Material
Acknowledgements
We would like to acknowledge Tommi Vähäsilta, MD, PhD and Mika Laine, MD, PhD for their essential contribution to the therapeutic course and the preparation of this article.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Funding: The article processing charges are provided from Helsinki Universtity Hospital research fund.
Contributor Information
Yoichi Sugiyama, Department of Cardiology, Heart and Lung Center, Helsinki University and Helsinki University Central Hospital, Haartmaninkatu 4, 00290 Helsinki, Finland; Department of Cardiology and Catheterization Laboratories, Shonan Kamakura General Hospital, 1370-1 Okamoto, Kamakura, Kanagawa 247-8533, Japan.
Noriaki Moriyama, Department of Cardiology and Catheterization Laboratories, Shonan Kamakura General Hospital, 1370-1 Okamoto, Kamakura, Kanagawa 247-8533, Japan.
Juho Viikilä, Department of Cardiology, Heart and Lung Center, Helsinki University and Helsinki University Central Hospital, Haartmaninkatu 4, 00290 Helsinki, Finland.
Pekka Raatikainen, Department of Cardiology, Heart and Lung Center, Helsinki University and Helsinki University Central Hospital, Haartmaninkatu 4, 00290 Helsinki, Finland.
Lead author biography
 Yoichi Sugiyama is an interventional cardiologist. He graduated from Nagoya City University in 2016. From 2021, he has worked at the Department of Cardiology and Catheterization Laboratories, Shonan Kamakura General Hospital, Japan. Currently, he is a research fellow in the Department of Cardiology, Heart and Lung Center, Helsinki University and Helsinki University Central Hospital, Finland.
Yoichi Sugiyama is an interventional cardiologist. He graduated from Nagoya City University in 2016. From 2021, he has worked at the Department of Cardiology and Catheterization Laboratories, Shonan Kamakura General Hospital, Japan. Currently, he is a research fellow in the Department of Cardiology, Heart and Lung Center, Helsinki University and Helsinki University Central Hospital, Finland.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. . Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 2. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. . Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 3. Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, et al. . Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol 2021;77:2717–2746. [DOI] [PubMed] [Google Scholar]
- 4. Fassa AA, Himbert D, Vahanian A. Mechanisms and management of TAVR-related complications. Nat Rev Cardiol 2013;10:685–695. [DOI] [PubMed] [Google Scholar]
- 5. Cook JR, Haery C, Montoya A. Potential contribution of open-cell stent design to balloon entrapment and review of techniques to recover. J Invasive Cardiol 2011;23:e183–e187. [PubMed] [Google Scholar]
- 6. Hiraya D, Sato A, Hoshi T, Sakai S, Watabe H, Ieda M. Incidence, retrieval methods, and outcomes of intravascular ultrasound catheter stuck within an implanted stent: systematic literature review. J Cardiol 2020;75:164–170. [DOI] [PubMed] [Google Scholar]
- 7. Piayda K, Hellhammer K, Veulemans V, Zeus T. Valvuloplasty balloon entrapment in a self-expanding aortic valve stent frame after inadvertent wire passage through the outflow struts. Catheter Cardiovasc Interv 2019;93:174–177. [DOI] [PubMed] [Google Scholar]
- 8. Eggebrecht H, Vaquerizo B, Moris C, Bossone E, Lämmer J, Czerny M, et al. . Incidence and outcomes of emergent cardiac surgery during transfemoral transcatheter aortic valve implantation (TAVI): insights from the European Registry on Emergent Cardiac Surgery during TAVI (EuRECS-TAVI). Eur Heart J 2018;39:676–684. [DOI] [PubMed] [Google Scholar]
- 9. Latson LA. Antegrade catheter snare for retrograde catheterization of the left ventricle: a new technique to facilitate balloon aortic valvuloplasty. Cathet Cardiovasc Diagn 1990;19:56–57. [DOI] [PubMed] [Google Scholar]
- 10. Naksuk N, Asirvatham SJ. Iatrogenic atrial septal defect: reassurance or inquisitiveness. J Interv Card Electrophysiol 2018;52:137–140. [DOI] [PubMed] [Google Scholar]
- 11. De Backer O, Landes U, Fuchs A, Yoon SH, Mathiassen ON, Sedaghat A, et al. . Coronary access after TAVR-in-TAVR as evaluated by multidetector computed tomography. JACC Cardiovasc Interv 2020;13:2528–2538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.