Figure 3. Hypoxia‐induced GDH1 acetylation was required for HIF1α stability.
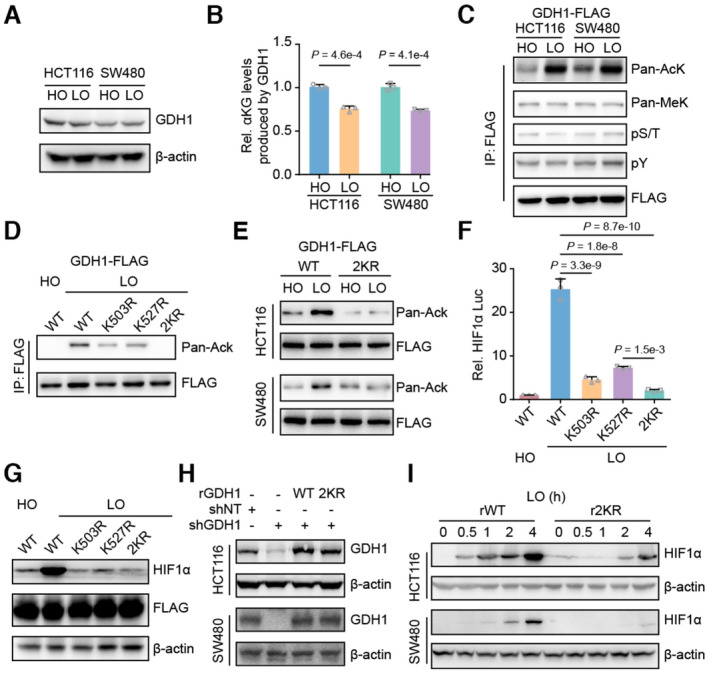
- HCT116 and SW480 cells were incubated under normoxia and hypoxia, respectively. Then, the cells were collected, and the GDH1 protein level was analyzed via immunoblotting.
- Hypoxia reduced the activity of GDH1 in producing αKG. A FLAG tag was inserted into the GDH1 C‐terminus in HCT116 and SW480 cells. The cells were collected after incubation under normoxia or hypoxia, and M2 flag beads were used to enrich FLAG‐tagged GDH1. GDH1‐FLAG was used to assess the enzymatic activity of GDH1 in producing αKG (n = 3).
- GDH1‐FLAG in (B) was examined using antibodies against pan‐lysine acetylation and methylation and pan‐serine/threonine and tyrosine phosphorylation.
- GDH1 acetylated at both K503 and K527 under hypoxia. K503R and K527R were mutated endogenously. GDH1‐FLAG was enriched, and an antibody against pan‐lysine acetylation was used to examine lysine acetylation of GDH1 when K503 or K527 was mutated to R503 or R527. 2KR represents K503/K527R.
- HCT116 or SW480 cells containing WT or GDH1 with K503/K527R mutations were incubated under normoxia and hypoxia, respectively. GDH1‐FLAG was enriched, and an antibody against pan‐lysine acetylation was used to test GDH1‐lysine acetylation.
- Loss of acetylation at GDH1‐K503 or GDH1‐K527 impaired the transcriptional activity of HIF1α (n = 3).
- HIF1α protein stability under hypoxia depends on GDH1‐K503 and GDH1‐K527 acetylation.
- GDH1‐depleted cell lines were constructed and then rescued with the expression of wild‐type GDH1 or K503/K527R mutant GDH1.
- HIF1α protein stability in rWT or r2KR mutant cells was induced over time under hypoxia.
Data information: Data are mean ± SD from the biological replicates (B, F). Statistics: unpaired two‐tailed Student's t‐test (B); one‐way ANOVA with Tukey's HSD post hoc test (F).
Source data are available online for this figure.
