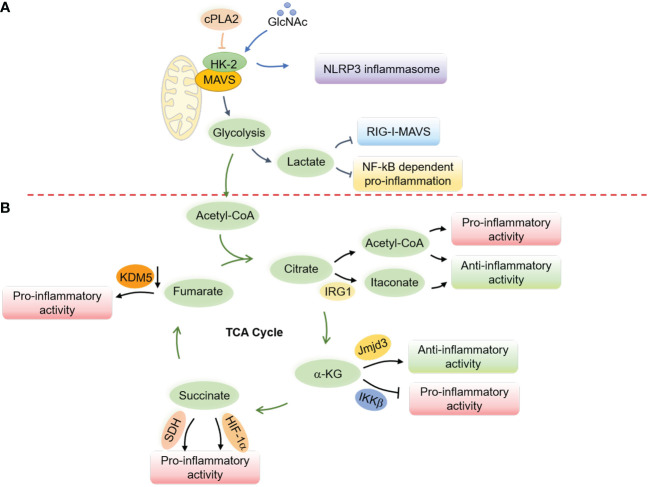
Figure 5.
Regulation of innate immune responses and macrophage activation by mitochondrial metabolism. (A) Glucose metabolism regulates the RLRs-MAVS signaling and the NLRP3 inflammasome through the hexokinase 2 (HK-2) and lactate generation. In a resting state, MAVS interacts with HK-2 to maintain its kinase activity and proper glycolysis process, while lactate production interrupts the mitochondrial localization of MAVS to suppress the RLRs-MAVS signaling. cPLA2 disrupts the interaction of MAVS and HK-2, thereby boosting NF-κB-related inflammation. GlcNAc, derived from the peptidoglycan of the bacterial cell wall, can interact with HK-2 to promote its redistribution into the cytoplasm and facilitate the activation of the NLRP3 inflammasome. (B) Metabolites from the TCA cycle control macrophage polarization by regulating chromatin modifications, DNA methylation, and post-translational modifications of proteins. Elevated cytosolic acetyl-CoA increases histone acetylation to promote the expression of inflammatory molecules and determine macrophage polarization. Itaconate, derived from cis-aconitate, engages anti-inflammatory activity in LPS-stimulated macrophages by inhibiting mtROS production, reducing succinate dehydrogenase (SDH) activity, blocking the inhibitor of NF-κB, the NF-κB-binding protein (IκBζ), and stabilizing nuclear factor erythroid 2-related factor 2 (Nrf2). α-KG engages anti-inflammation via Jmjd3-dependent metabolic and epigenetic reprogramming and inhibits the proline hydroxylation of IKKβ to repress pro-inflammation. Succinate facilitates proinflammatory activity by enhancing ROS production through stabilizing HIF-1α and being oxidized by SDH. Fumarate increases proinflammatory activity by inhibiting the KDM5 histone demethylase activity, thereby promoting the gene transcription of TNFα and IL-6 cytokines.