Abstract
Polymer foams (PFs) are among the most industrially produced polymeric materials, and they are found in applications including aerospace, packaging, textiles, and biomaterials. PFs are predominantly prepared using gas-blowing techniques, but PFs can also be prepared from templating techniques such as polymerized high internal phase emulsions (polyHIPEs). PolyHIPEs have many experimental design variables which control the physical, mechanical, and chemical properties of the resulting PFs. Both rigid and elastic polyHIPEs can be prepared, but while elastomeric polyHIPEs are less commonly reported than hard polyHIPEs, elastomeric polyHIPEs are instrumental in the realization of new materials in applications including flexible separation membranes, energy storage in soft robotics, and 3D-printed soft tissue engineering scaffolds. Furthermore, there are few limitations to the types of polymers and polymerization methods that have been used to prepare elastic polyHIPEs due to the wide range of polymerization conditions that are compatible with the polyHIPE method. In this review, an overview of the chemistry used to prepare elastic polyHIPEs from early reports to modern polymerization methods is provided, focusing on the applications that flexible polyHIPEs are used in. The review consists of four sections organized around polymer classes used in the preparation of polyHIPEs: (meth)acrylics and (meth)acrylamides, silicones, polyesters and polyurethanes, and naturally occurring polymers. Within each section, the common properties, current challenges, and an outlook is suggested on where elastomeric polyHIPEs can be expected to continue to make broad, positive impacts on materials and technology for the future.
1. Introduction
Polymer foams (PFs) are materials consisting of a polymeric network with gas-filled voids. The PF market is projected to reach over USD $160 billion by 2023 and expected to exceed a volume of production over 18 kilotons by 2026.1−3 PFs are prepared from both thermoplastic and thermoset polymers, and PFs can be highly rigid or flexible depending on the chemistry of the polymer network, making them suitable for use in many industries including automotive, textiles, aerospace, and biomaterials.4,5 PFs are predominantly prepared industrially by batch- or extrusion-production methods using physical- or chemical-foaming techniques.6 These types of PFs are described as “gas-blown” foams. Physical-foaming methods rely on dissolving gas within a polymer that is heated above its glass transition temperature (Tg) in a pressurized environment. The pressure is rapidly released while the thermoplastic is still heated, causing an expansion of the dissolved gas and causing the polymer foam to form. These types of PFs are made from high-molecular-weight thermoplastics including poly(methyl methacrylate) and polyethylene and gases including CO2, N2, and H2. Alternatively, chemical-foaming techniques are primarily used for thermosetting polymers, which are foamed using an internal or additive chemical compound that produces gas when exposed to a stimulus, such as heat, during the curing process.
As an alternative to gas-blowing methods, PFs can be prepared by synthesizing a polymer network around an immiscible template, where the immiscible phase can be either a solid or liquid. When a solid particle is used as the template, the method is known as particulate leaching, and this method has often been used in biomaterial applications.7−10 When a liquid phase is used as the template, the materials are called emulsion-templated foams. Emulsion-templated PFs obtain their porosity, pore size, pore structure from the droplet structure of the precursor emulsion template. When the internal phase (the immiscible template) of the emulsion reaches a volume fraction over 74%, the resulting foams are conventionally called polymerized high internal phase emulsions, or polyHIPEs.11,12 Polymer foams prepared using templated emulsions that possessed internal phase volume fractions of lower than 74% are classified as medium internal phase emulsions (MIPEs) when the volume fraction is between 30 and 74% and low internal phase emulsions (LIPEs) when the volume fraction is less than 30%. Throughout this review, we will use the term “polyHIPE” when discussing the technique or in general terms as most of the examples can be classified as such, but specific examples will be described by their formal name with respect to the internal phase volume fraction when necessary. The emulsion is prepared by the stabilization of an internal (or dispersed) phase within a continuous phase consisting of polymerizable monomers and, if required, cross-linkers. The emulsion then undergoes a polymerization process followed by subsequent removal of the internal phase to yield a polymer foam (Figure 1).
Figure 1.
Cartoon overview of a water-in-oil emulsion template to prepare a porous monolith. From left to right, an emulsion is prepared, polymerized, and purified to remove the dispersed phase and produce a polyHIPE shown by the SEM image (far right).
Additionally, either open-cell or closed-cell pore structures can be achieved by in polyHIPE PFs either by controlling the locus of initiation of polymerization via choice of initiator or through the type of surfactant used to stabilize the emulsion.13 Generally, open-cell polyHIPEs can be achieved by initiating the polymerization from the continuous phase of the emulsion, while closed-cell polyHIPEs can be obtained by initiating the polymerization from the internal phase or from Pickering emulsions using a particle-based surfactant. Recent review articles from Foudazi14 and from Silverstein and co-workers15 provide comprehensive overviews on controlling polyHIPE pore morphology.
PolyHIPEs can possess a range of mechanical properties, as the stiffness of the polyHIPE is dependent on the polymers in the polymer network. Rigid polyHIPEs are have been widely reported and can be traced back to the early reports of polyHIPEs prepared from the free radical polymerization of styrene (S) with divinylbenzene (DVB) as a cross-linker.16,17 Since then, rigid polyHIPEs have remained commonly prepared from S/DVB-based copolymer networks and can be found in applications including liquid and gas separation membranes, heterogeneous supports, and low-density thermal insulators.18−21 Similarly, rigid polyHIPEs can also be prepared from polar monomers, resulting in hydrophilic surfaces that are useful in biomaterial applications such as bone tissue engineering. A recent review from Claeyssens and Dikici22 provides a more detailed overview of polyHIPEs for tissue engineering applications than we can provide here. These types of applications rely on the material to resist deformation or degradation under harsh working conditions such as corrosive and high-temperature environments and maintain the intended high surface and porosity.
In addition to rigid polyHIPEs, there is a need for elastomeric polyHIPEs that can be deformed under mechanical force and recover their original structure over many cycles without the loss of the porous structure. These types of polyHIPEs have been historically less common, but the preparation of these flexible polyHIPEs can give rise to new materials that can address challenges in the fields of soft electronics, 3D tissue engineering, and many others where rigid polyHIPEs fail. This review provides an overview of elastomeric polyHIPEs and the chemistry used to synthesize these materials. We have organized the polyHIPEs into categories based on the chemistry of the polymer network, and within each type of polymer network the proposed applications of these materials have been explored.
2. (Meth)acrylics and (Meth)acrylamides
Many elastomeric polyHIPEs consist of copolymer networks prepared from acrylic- and methacrylic-based monomers with long hydrocarbon side chains using free radical polymerizations. These monomers have high side chain mobility and are known to plasticize polymer networks, introducing elasticity.23,24 Thus, introducing comonomers having long hydrocarbon side chains to the classic styrene (S)–divinylbenzene (DVB) polyHIPE networks yielded polyHIPEs that were elastic and compressible rather than brittle. These polyHIPEs were typically prepared from water-in-oil emulsions and possessed properties including controlled thermomechanical properties and resistance to many chemical environments, making them suitable for applications such as shape memory foams and separation membranes. Elastic polyHIPEs can also be prepared from copolymer networks of acrylic-based monomers that have polar functional groups such as hydroxyls, amines, and ionic moieties. These polyHIPEs are commonly prepared from oil-in-water emulsions to produce emulsion-templated hydrogels, or polyHIPE-hydrogels, and they have been used in applications including tissue engineering and absorbents.
2.1. Shape Memory Foams
PolyHIPEs prepared with a flexible comonomer in the polymer network result in porous materials with a much lower glass transition temperature than when using the traditional S/DVB monomers. For example, one of the first elastic polyHIPEs was reported by Cameron and Sherrington,25 and they showed that addition of 2-ethylhexyl acrylate (EHA) or 2-ethylhexyl methacrylate (EHMA) to S/DVB polyHIPEs imparted tunable Tgs and elasticity without impacting the pore size, pore shape, or pore interconnectivity in the polyHIPEs over the comonomer ratios tested. In that work, the Tg of the S/DVB polyHIPEs could be decreased from ∼100 °C to as low as −10 and −50 °C with blends of EHMA or EHA, respectively.25 Ulubayram and co-workers26 showed that the ultimate compression strength and Young’s modulus (E) of DVB-based polyHIPEs were dependent on the architecture of the comonomer side chains. For example, stearyl acrylate polyHIPEs were elastic and had a Young’s modulus of ∼2.5 MPa at a monomer:DVB ratio of 60:40 compared to more rigid isobornyl methacrylate based polyHIPEs that possessed a Young’s modulus value of ∼12 MPa.26
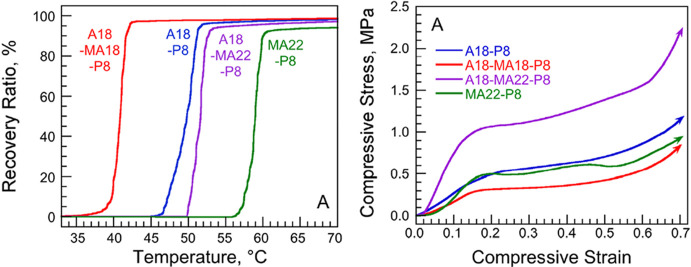
Since this report, EHA and other long-side-chain (meth)acrylates have been used as plasticizing comonomers in DVB systems to yield shape memory polyHIPEs. For example, Silverstein and co-workers27 showed thatshape memory polyHIPEs could be achieved with various long-side-chain (meth)acrylates (Figure 2). The Tm could be tuned from ∼35 to 55 °C while maintaining high shape recovery (>85%) using different formulations of stearyl (meth)acrylate and behenyl (meth)acrylate with acrylate-functionalized silsesquioxane and DVB as a cross-linker.27
Figure 2.
Recovery ratio versus temperature of polyHIPEs stabilized with silica nanoparticles and star surfactants having different comonomer and cross-linker ratios (left) and the corresponding compressive stress–strain curves (right). In each plot, the data are named according to their preparation formulation where A18 and MA18 are stearyl (meth)acrylate, MA22 is behenyl methacrylate, and P8 is acrylate-functionalized silsesquioxane. Reprinted with permission from ref (27). Copyright 2021.
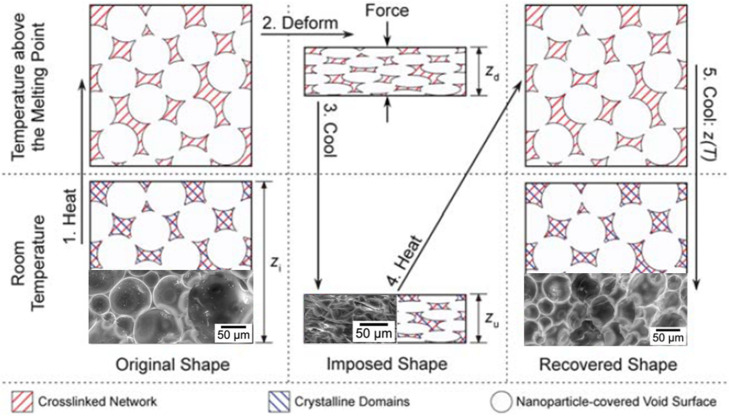
In addition to copolymerizations, polyHIPEs synthesized from fully methacrylate-based networks have shown to have shape memory characteristics. Specifically, Silverstein and Gurevitch28 prepared polyHIPEs using the homopolymerization of stearyl (meth)acrylate with methacrylate functionalized silica nanoparticles as cross-linkers and stabilizers in a water-in-oil Pickering emulsion (Figure 3). PolyHIPEs made from acrylate networks had two crystallinity phases with melting temperatures of ∼47 and 52 °C, while polyHIPEs with a methacrylate polymer backbone functionality had only one at ∼29 °C. Both polyHIPEs had shape fixity of 100% at room temperature and ∼90% shape recovery over four compression–recovery cycles.28
Figure 3.
Schematic overview of the shape memory testing that was performed on stearyl acylate and methacrylate based polyHIPEs. The inset SEM images show the porous structure before (left), during (middle), and after (right) a compressive deformation of 65%. Reprinted with permission from ref (28). Copyright 2012.
2.2. Oil Absorbents and Separation Membranes
The flexible, porous, and nonpolar nature of acrylic-based polyHIPEs prepared from long-side-chain monomers allow them to reversibly swell in nonpolar mediums and perform as a sponge. For example, Hanabusa and co-workers29 showed that a wide range of organic solvents could be absorbed by polyHIPEs prepared from n-BA, EHA, and methyl methacrylate (MMA) with 1,6-hexanediol diacrylate as a cross-linker. The swelling ratio was dependent on the type of monomer selected, with polyHIPEs prepared from EHA obtaining the highest swelling ratio of ∼330% in THF with a capacity of ∼30 g/g. Additionally, blends of EHA and MMA produced mechanically robust polyHIPEs that could reversibly swell and eject the solvent after compression for more than 20 cycles without a significant loss in performance.29
The thermal and chemical and thermal resistance of acrylate-based polyHIPEs can be improved by preparing composite polyHIPEs by introducing additives such as siloxanes, which can also impart superhydrophobicity.30 For example, Ngai and co-workers31 showed that using silica nanoparticles as a Pickering emulsifier and polydimethylsiloxane (PDMS) as an additive to an n-BA polyHIPE introduced hydrophobic and oleophilic properties as a self-cleaning foam (Figure 4).
Figure 4.

(a) Digital images of a superhydrophobic n-BA-based polyHIPE that showed superhydrophobicity to a variety of complex aqueous media (left) and resistance to strong acids and bases (right). (b) Digital images of self-cleaning capabilities in air (top) and underwater (bottom). Reprinted with permission from ref (31). Copyright 2020 American Chemical Society.
Functionalization of polyHIPE surfaces can be achieved by introducing reactive comonomers such alkenes from a bio-based feedstock such as myrcene,32 or more commonly as epoxides from glycidyl methacrylate (GMA).33 For example, Krajnc and co-workers34 showed that polyHIPEs prepared from copolymer networks of EHA and GMA could be ring-opened using diamines in a postpolymerization process to yield amine-functionalized pore surfaces. The amine-functionalized polyHIPEs showed high protein binding capacities and could separate myoglobin, conalbumin, and trypsin when prepared into a column as an ion-exchange surface. Importantly, the polyHIPEs maintained their elasticity over the application conditions, having a compressive modulus of ∼2 MPa and a maximum compressive strain of ∼20% when 10 wt % EHA was used.
2.3. Tissue-Engineering Scaffolds
PolyHIPEs prepared from water-in-oil emulsions from monomers containing a degradable functional group, for example an ester, have been used in tissue-engineering applications.35 The Cosgriff-Hernandez group developed a series of shelf-stable injectable polyHIPEs as degradable bone scaffolds from dimethacrylate-functionalized propylene fumarate (PFDMA),36−38 ethylene glycol dimethacrylate,37 and butanediol dimethacrylate.37 Due to the stability of the HIPE templates, the emulsions could be stored in a syringe for up to 6 months before polymerization, producing polyHIPEs with compressive moduli ranging from 15 to 40 MPa and compressive strengths of 1–5 MPa.37 A fully interconnected pore morphology in the PFDMA-based polyHIPEs was achieved by shifting the locus of initiation to the continuous phase of the water-in-oil emulsion by using the oil-soluble initiator benzoyl peroxide.38 This highly interconnected pore structure aids in fluid transport and cell movement through the scaffold, and the biodegradable nature of PFDMA make these polyHIPEs suitable bone-tissue-engineering scaffolds. The use of photoinitiated polymerizations of acrylic-based polyHIPEs are increasingly being used in photocured 3D printing for better control over the design of bone scaffolds, and SEM images of the typical printed polyHIPEs can be found in Figure 5.39−42 Emulsions, especially HIPEs, are inherently viscous, making them ideal candidates for deposition-based stereolithographic additive manufacturing techniques where limited spreading after deposition is required.43
Figure 5.
Examples detailing the typical macro- and microstructures obtained through photo-3D printing of emulsions. (a) SEM images from a polyHIPE that was 3D printed where the cross-hatched woodpile design with high print resolution is obtained (left) while maintaining the spherical pore structure from the emulsion template (right). Reprinted with permission from ref (39). Copyright 2016. (b) Overview of the cure-on-dispense method used to prepare 3D-printed polyHIPEs (left) that had highly interconnected spherical pores in the inset SEM image (right). Reprinted with permission from ref (41). Copyright 2016.
In addition to the porous microstructure obtained from printing polyHIPEs, custom macroscopic structures including voids, layers, and struts can be designed using additive manufacturing processes. Control over both these micro- and macro-structures has been shown to be beneficial in the preparation of the complex architectures needed for bone tissue engineering.44 For example, Claeyssens and co-workers39 prepared a series of 20 different woodpile-design polyHIPEs having porosity values of 75–90% using a direct-write stereolithographic approach (Figure 5a). Elongation and ultimate tensile stress were controlled by changing the composition of EHA and isobornyl acrylate (IBOA) monomers with trimethylolpropane triacrylate as the cross-linker. It was highlighted in this work that the challenges associated with large (over 1 mm thick) 3D monolithic shapes, such as inhomogeneous plasma treatment and limited cell penetration, could be overcome by 3D printing polyHIPEs in a woodpile design with struts having diameters of ∼300 μm.
Similarly, emulsion-templated hydrogels with macroscopic porosity can be prepared with improved biological benefits compared to traditional hydrogel analogues, including improved cell ingrowth and nutrient diffusion due to the polyHIPE highly interconnected pore morphology leading to improved cell survival.45 These polyHIPE hydrogels have been used in soft tissue applications. For example, Qureshi and co-workers46 showed that the cell viability of NIH/3T3 fibroblast cells was dependent on the surface area and pore interconnectivity of elastic polyHIPE hydrogels prepared from glycerol monomethacrylate/2-hydroxyethyl methacrylate (GMMA/HEA) copolymer blends from oil-in-water emulsions (Figure 6). The static compressive strain of the hydrated polyHIPE hydrogels at a constant load of ∼10 kPa could be tuned from ∼25 to 40% by changing the ratio of GMMA to HEA, where the material with the highest HEA content (60 wt %) had the highest compressive strain and strain recovery of 92% after the load was released.46
Figure 6.
(a) Synthetic route to preparing polyHIPE hydrogels from oil-in-water emulsions (left) and corresponding SEM images of the dried polyHIPE hydrogels where the GMMA content is increased from A to D (right). (b) Fluorescence microscope images of NIH/3T3 cells seeded after 7 days on a control scaffold (top left) and polyHIPE hydrogels with increased amounts of GMMA content from left to right where the number of live cells (green) are higher than that of dead cells (red). Reprinted with permission from ref (46). Copyright 2016.
2.4. Aqueous Absorbents and Ionic Molecule Capture
Elastic PolyHIPEs consisting of highly hydrophilic or charged polymer networks have been prepared that have properties including superswelling in aqueous environments and have been used in applications including reinforced hydrogels,47 soil-free plant growth,48 and aqueous absorbents.49 For example, Kovačič and co-workers48 prepared a polyelectrolyte polyHIPE from (3-acrylamidopropyl)-trimethylammonium chloride (AMPTMA) that showed water uptake as high as ∼35 g/g that could support growth of chickpea roots through the media (Figure 7). Similarly, Silverstein and Kovačič50 prepared a series of polyelectrolyte polyHIPEs from 2-acrylamido-2-methyl-propanesulfonic acid (AMPS) and N,N′-methylenebis(acrylamide) that showed a water uptake capacity as high as ∼340 g/g, which is higher than that of the commercial absorbent (SAP powder) used in diapers.
Figure 7.

(a) Digital image showing the dry (left) and equilibrium water swollen AMPTMA-based polyelectrolyte polyHIPE (right). (b) SEM image of the porous structure of the dried polyelectrolyte polyHIPE (left) and digital images of the polyHIPE supporting growth of a chickpea plant compared to an agar control. Modified with permission from ref (48). Copyright 2022 American Chemical Society.
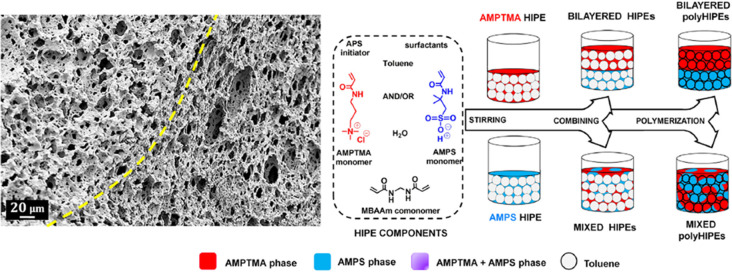
In addition to high water uptake, the high surface area and porosity of polyelectrolyte polyHIPEs make them an ideal candidates for the removal of charged contaminants from aqueous media. Specifically, Žagar and co-workers51 prepared cationic-polyelectrolyte polyHIPEs from an oil-in-water emulsion containing acrylamide-based monomers AMPTMA and N,N′-methylenebis(acrylamide) (MBAAm) to capture erythrosine dye as a model for anionic contaminants. The high porosity (∼80%) coupled with the cationic nature of the polymer network resulted in excellent water uptake capabilities (95 g/g) and erythrosine dye removal of ∼95% in under 3 h. Subsequently, Kovačič and co-workers52 prepared amphoteric polyHIPE hydrogels using a two-emulsion layering technique of emulsions consisting of AMPS or AMPTMA (Figure 8). Amphoteric polyHIPE hydrogels with defined layers of the cation and anionic networks showed removal of ∼70% of both eosin and methylene blue dyes from water in 48 h compared to nonlayered copolymer networks having only 27% removal. This improved performance for layered systems is attributed to the spatially separated polyelectrolyte networks limiting the screening of charges that occurred for the nonlayered polyHIPE hydrogels.
Figure 8.
Overview of the layering method used to prepare layered, mixed, and copolymer polyHIPE hydrogels. SEM image of a dry layered material where the interface of the cationic and anionic portions is highlighted with a yellow dashed line (far left). Reprinted with permission from ref (52). Copyright 2020.
The expanding list of (meth)acrylate- and (meth)acrylamide-based monomers shown to be compatible with emulsion-templated polymerizations has developed a library of elastomeric polyHIPEs having properties that are useful in a range of applications. The current resins used in stereolithographic additive manufacturing are mainly acrylic,s making 3D printing an area suitable for the continued growth of acrylate-based polyHIPEs.
3. Silicones
Polydimethylsiloxane (PDMS) is used to prepare porous elastomers for applications including biomaterials, electronics, and microfluidic devices.53 The most common method to prepare PDMS elastomers is using organoplatinum catalysts (e.g., Karstedt’s catalyst) in hydrosilylation reactions between vinyl- and hydrosilane-containing PDMSs. The first reported open-cellular PDMS monolith from an emulsion template using this type of reaction was prepared using a water-in-PDMS (vinyl- and hydrosilane-containing PDMS) emulsion. Deleuze and co-workers54 prepared polyHIPEs possessing total porosity values of ∼80%, and the compressive Young’s modulus was found to be dependent on the cross-linking density (Si–vinyl to Si–H ratio) in the HIPE formulation and ranged from 4 to 8 MPa over the ratios tested. Similarly, early examples of porous PDMS particles used water-in-PDMS-in-water double-emulsion techniques to prepare elastic particles with tunable pore sizes and density through hydrosilylation reactions of PDMSs.55,56
3.1. Separation Membranes
PolyHIPEs have been prepared from hydrosilylation reactions using commercial two-part resin kits (e.g., Sylgard, Solaris, and others) that have been widely used as separation membranes to remove dyes, organic solvents, and petroleum derivates from aqueous environments.57 The polyHIPEs prepared from these standard resin kits possess high porosities, are hydrophobic, and are resilient to mechanical deformation, making them ideal materials as reusable separation membranes. For example, Liu and co-workers58 used a simple and scalable method to prepare PDMS-based polyHIPE sponges that could uptake gasoline, chloroform, and other water contaminants with the highest uptake capacity being ∼35 g/g and maintained ∼95% capacity over more than 50 recovery cycles. To impart more targeted properties, these resin kits can be modified with additives such as metallic particles or additional modification steps to prepare PDMS-polyHIPE composites with multiple functionalities.59 Fragouli and co-workers60 showed a PDMS-polyHIPE made from Sylgard could be coated with polydopamine (PDA) and then decorated with silver nanoparticles (AgNP) to introduce both underwater oleophobicity and antimicrobial properties to the separation membrane (Figure 9). The AgNP/PDA-coated polyHIPEs showed a high oil rejection of over 99% and, due to the AgNP available at the surface, showed high antibacterial properties to E. coli bacteria of filtered media, where no bacterial growth was seen in overnight studies.
Figure 9.
(a) Cartoon overview of the sequential polymerization and functionalization steps to prepare silver-coated PDMS polyHIPEs. (b) Oil rejection efficiency. (c) E. coli bacterial optical density versus time plot. (d) Digital image of a Petri dish containing filtered aqueous media contaminated with E. coli from overnight growth. Reprinted with permission from ref (60). Copyright 2021.
Alternatively, iron oxide particles can be introduced to the PDMS network to prepare polyHIPE particles that can be easily recovered from the environment using a magnet.61 In this work, a water-in-PDMS-in-water double emulsion was prepared and cured to produce PDMS-polyHIPE particles with sizes of ∼40–60 μm and had swelling capacities of ∼400% in heptane.
3.2. Flexible Sensors
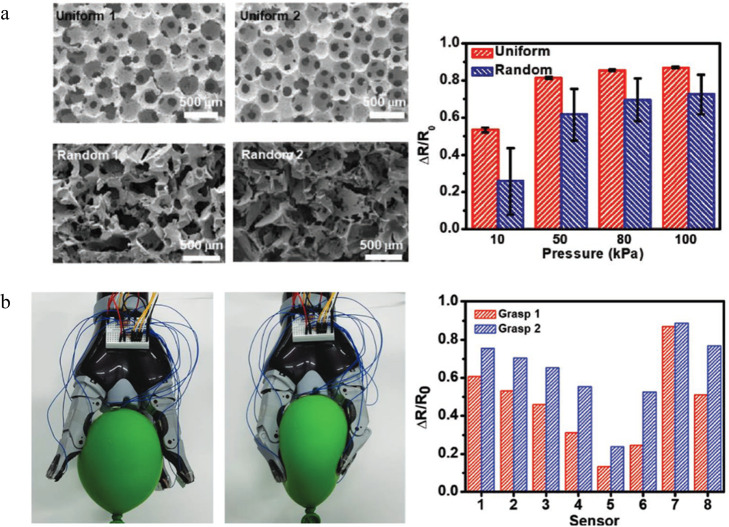
Recently, wearable sensors have been prepared from PDMS-based polyHIPEs by introducing conductive additives such as carbon-based nanomaterials62−64 or polypyrrole65 to PDMS precursor resins. Applying mechanical forces (i.e., bending, compressing, or stretching) to conductive polyHIPEs causes an electric response due to the piezoelectric effect. For example, Park and co-workers65 used a microfluidic approach to synthesize monodisperse water-in-Sylgard emulsions that were polymerized and subsequently functionalized with polypyrrole (Figure 10). These polyHIPEs showed a rapid sensor recovery time of ∼60 ms with high sensitivity and a low limit of detection, obtaining values of 70 and 80 Pa, respectively. It was highlighted in this work that the fluidics-based polyHIPEs showed negligible hysteresis in the relative resistance over 1000 cycles and a minimal coefficient of variance between sensors due to the highly uniform pore structure compared to controls with a random pore structure.
Figure 10.
(a) SEM images of microfluidics-based polyHIPEs versus random orientation (left) and the corresponding sensor relative resistance change for each sensor at different pressures (right). (b) Digital images of a robotic gripper with sensors integrated into the fingers (left) and the relative resistance change during light and strong squeezing. Reprinted with permission from ref (65). Copyright 2019.
In addition to these conductive networks, PDMS-polyHIPEs can also act as the dielectric layer between two electrodes in pressure sensors when fabricated into arrays of pillar structures66,67 or hierarchically porous films.68 For these types of sensors, the soft, compressible polyHIPE is the key component in obtaining high sensitivity to applied pressure, as the slight changes in distance between the two electrodes when the polyHIPE layers deforms can be recorded.
3.3. Soft Acoustic Materials
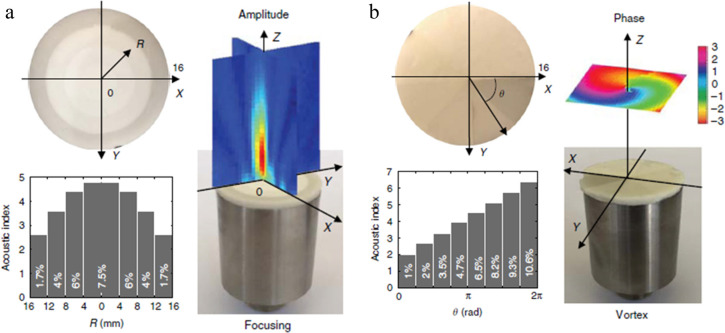
PDMS-based polyHIPEs have been synthesized from epoxy- and thiol-containing PDMS for acoustic applications. In acoustic applications, the longitudinal sound speed (cL) through a material depends on the density and the bulk modulus (K) of the material, making soft, porous material PDMS polyHIPEs excellent candidates for study. The Mondain-Monval and Brunet groups have prepared a library of epoxy-PDMS polyMIPE and polyLIPE monoliths and monodisperse porous beads with low porosity (<40%) that can control longitudinal sound speeds.69−71 Specifically, porous PDMS beads with a diameter of 300 μm and a porosity of ∼20% were prepared through emulsion microfluidics that when suspended in a support matrix obtained a negative acoustic index over ultrasonic frequencies.72 In related work, flat acoustic waveguides were prepared from the same PDMS network by patterning monoliths with controlled acoustic indices in a gradient fashion to focus, spin, or bend acoustic waves (Figure 11).73 Total porosity was controlled for each monolith portion from 0% to 15%, resulting in relative refractive acoustic indices from 1.4 to 7.5.
Figure 11.
Digital images of flat acoustic waveguides in (a) a bullseye and (b) flat azimuthal pattern from a top view (top left in each) and the corresponding acoustic indices for the individual layers (bottom left in each) and digital images of the waveguides located on a transducer (bottom right in each) with the measured acoustic phase field heat map overlaid (top right in each). Reprinted with permission from ref (73). Copyright 2019.
Our lab has synthesized PDMS polyMIPE polyHIPEs with tunable porosity and storage moduli using thiol- and ene-functionalized PDMSs.74,75 Recently, we reported76 thiol–ene PDMS polyMIPEs that resulted in low longitudinal sound speeds of ∼40 m/s through the polyMIPEs at ultrasonic frequencies. The storage modulus (G′) was dependent on the thiol to ene ratio of the PDMS components and polyMIPEs, and G′ values from ∼40 to 230 kPa could be obtained at a single porosity of ∼40%.
3.4. Fluid-Filled Elastomers
In addition to the more common open-cell porous morphology, closed-cell PDMS-polyHIPEs have been prepared using Pickering emulsions where the gas or liquid is intentionally contained within the network. For example, Bismarck and co-workers77 used an aqueous sodium hydrogen carbonate solution as the dispersed phase in a closed-cell PDMS polyHIPE to act as a pressure-sensitive blowing agent. The evolved CO2 gas trapped in the monolith expanded upon reduced pressure, resulting in materials with volume expansion of up to ∼30 times without total rupture of the surrounding PDMS network. Maintaining the dispersed phase liquid within the PDMS-polyHIPE has been used in applications such as ferrofluid-filled magnetic elastomers,78 liquid-reinforced elastomers,79 and synthetic plant tissue.80 In synthetic plant tissue analogues (PTA), the initial dispersed phase consisted of aqueous salt solutions of various salt concentrations that experienced osmotic-induced swelling when submerged in pure water (Figure 12).81 The swelling properties of these liquid-filled elastomers were dependent on the polyHIPE wall thickness and the salt concentration of the encapsulated aqueous phase, and they were strong enough to lift a weight fixed to the end of the PTA.
Figure 12.
(left) Schematic overview of osmotic-induced swelling behavior of synthetic plant tissue synthesized from a closed PDMS polyHIPE and (right) actuation motion of a PTA compared to a standard poly(acrylic acid) hydrogel over a loading and unloading cycle. Reprinted with permission from ref (81). Copyright 2021.
Off-the-shelf silicone resin kits have proven to be simple and efficient precursors for the preparation of elastic polyHIPEs. One can expect to see more advances in reusable separation membranes, and future studies will continue introducing novel functionalities to the pore surfaces and networks. One challenge that persists regarding silicone-based open-cell polyHIPEs is the inability to maintain high (>90%) total porosity during removal of the dispersed phase without the use of internal blowing agents (H2, H2O2, CO2) or advanced drying techniques like supercritical CO2. New approaches need to be realized to prepare more rigid, but still elastic, silicone networks. Routes targeting highly cross-linked silicones or localized reinforcement of the pore wall can be imagined.
4. Polyesters and Polyurethanes
Porous polyester (PE) and polyurethane (PU) materials are among the most produced porous polymers industrially and can be found in applications including biomaterials, textiles, automotives, and packaging.3,5 Emulsion-templated PEs and PUs have been mainly prepared by two routes. The first route relies on a multistep approach where a PE or PU is first polymerized and isolated, and then an emulsion can be prepared using the precursor as a cross-linkable component in the emulsion. The end group of the isolated PE or PU is then functionalized, typically to acrylate, acrylamide, or other terminal alkene groups, to be compatible in free radical polymerizations with other acrylate-containing comonomers. We have separated the discussion of these PE and PU polyHIPEs from the fully acrylic-based polyHIPEs in Section 2, as the defining properties of these PE- or PU-based materials are from the PE or PU in the polyHIPE. The second, less common, route consists of the direct polymerization and cross-linking of PE or PU precursor monomers within the continuous phase of an emulsion in one step. For this route, the emulsions are typically oil-in-oil emulsions because water is known to cause unwanted side reactions in PE and PU polymerizations. Both routes can successfully prepare elastomeric polyHIPEs that can be used as degradable scaffolds in tissue engineering, conductive networks, and absorbents.82
4.1. Tissue-Engineering Scaffolds
PolyHIPEs consisting of polyesters, polyurethanes, or copolymer blends are predominantly found in tissue-engineering applications as degradable scaffolds. Polycaprolactone (PCL) is a degradable ester-functionalized polymer that is has been used as a biomaterial in some applications that have been FDA-approved, and porous PCL-based scaffolds have been prepared using emulsion-templating techniques. Specifically, one of the first reported degradable polyHIPEs was a copolymer blend of styrene or methyl methacrylate with PCL-diacrylate. Jahoda and co-workers82 prepared PCL-diacrylate with different molecular weights (Mn of ∼700 or 2000 g/mol) as a degradable cross-linker that could be copolymerized with either S or MMA in free radical polymerizations. The swelling ratio of the polyHIPEs depended on the amount and molecular weight of the PCL-diacrylate component, and the styrene-based polyHIPE with 20 wt % of the higher molecular weight PCL could support human fibroblast cells over 2 days; this result was confirmed by an imaging analysis performed after staining the cells with Giemsa.
Since that work, there have been reports showing elastic polyHIPEs with PCL contents as high as 50 wt % prepared through similar multistep approaches using various acrylate-functionalized PCL precursors with comonomers such as S, EHA, and tert-butyl acrylate (t-BA).83,84 In those examples, Silverstein and co-workers established that the amount of PCL in the network was used to control the elastic properties of the polyHIPEs while maintaining high degradability and an open-cell pore morphology. Specifically, polyHIPEs prepared with 10 wt % PCL-diacrylate and styrene as a comonomer obtained an elastic modulus of 2.8 MPa while polyHIPEs prepared with 50 wt % PCL-diacrylate had a much lower elastic modulus of 760 kPa (Figure 13). Additionally, the elastic modulus could be controlled by selecting different comonomers. For example, polyHIPEs prepared with 50 wt % PCL-diacrylate and t-BA as a comonomer obtained the lowest elastic modulus of ∼12 kPa under compression testing compared to polyHIPEs prepared with EHA that obtained an elastic modulus of ∼70 kPa.
Figure 13.
(a) SEM images of PCL/S polyHIPEs with 10 wt % (left) and 50 wt % (right) PCL contents. (b) Stress–strain plot comparing polyHIPEs using either DVB or PCL as a cross-linker where B10 is 10 wt % DVB and L10 and L25 are 10 and 25 wt % PCL, respectively (left), and degradation studies of PCL-polyHIPEs with either EHA or S as comonomers (right). Reprinted with permission from ref (84). Copyright 2009.
In addition to copolymer blends, fully PCL-based polyHIPEs can be prepared from end functionalization of PCL to photopolymerizable methacrylate groups as first reported by Claeyssens and co-workers.85 In related work, Claeyssens and co-workers86 prepared fully PCL-based polyHIPEs from the homopolymerization of a 4-arm PCL-methacrylate prepolymer (Figure 14). The polyHIPEs obtained a highly interconnected pore structure with an average pore size of ∼35 μm and remained elastic upon elongation up to ∼90%. The polyHIPEs were subsequently functionalized using a fibroblast-derived extracellular matrix to enhance the biocompatibility of the scaffold, resulting in improved angiogenesis of chick chorioallantoic membrane in the area of implantation in vivo compared to nonfunctionalized control polyHIPEs.
Figure 14.
(a) Schematic overview of the cellularization biofunctionalization process for PCL-based polyHIPEs. (b) Digital images of the implanted PCL-tissue scaffolds (left) and corresponding quantification of blood vessels from the control or functionalized PCL-polyHIPE showing improved angiogenesis. Reprinted with permission from ref (86). Copyright 2021.
PCL can be used as a flexible synthetic polymer to improve the mechanical properties of porous hydrogels prepared from naturally occurring polysaccharides and peptides, such as chitosan and gelatin, respectively.87 Specifically, the use of double networks (DN) in hydrogels consisting of a rigid network and a flexible network can improve the elasticity and toughness of the hydrogel. For example, Dehghani and co-workers88 reported the synthesis of DN chitosan/PCL polyLIPE/MIPE hydrogels for 3D tissue-engineering applications. The mechanical properties of the DN hydrogels were significantly improved compared to single-network chitosan hydrogels, obtaining a compressive modulus of ∼33 kPa at 50 wt % PCL compared to ∼9 kPa for pure chitosan hydrogels, both at 50% strain in the hydrated state.
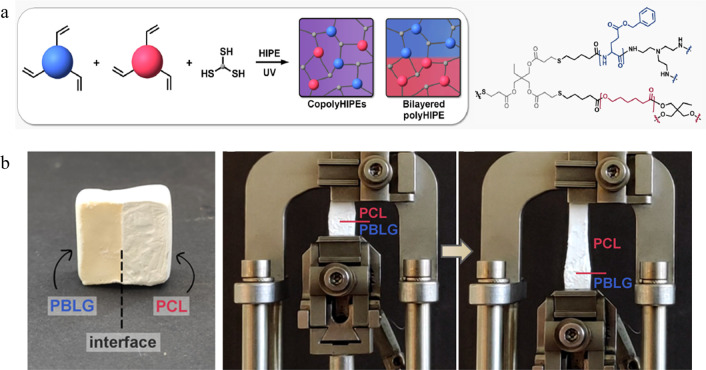
Degradable polyHIPEs prepared from copolymer network blends of esters,89 urethanes,90−92 and peptides have been prepared. Specifically, one method used to prepare polyHIPEs with these types of blended copolymer networks is a thiol–ene click reaction between (meth)acrylate-functionalized prepolymers.93,94 For example, Pahovnik and co-workers95 synthesized polyHIPEs with tunable mechanical properties by varying the ratio of a rigid poly(benzyl-glutamate) polypeptide (PBLG) with PCL, a more flexible polyester (Figure 15). The compressive modulus and ultimate stress of the polyHIPEs were dependent on the PBLG content. Pure PCL polyHIPEs had a compressive modulus of ∼0.2 MPa, with increasing moduli being observed with increasing PBLG content in the polyHIPEs until pure PBLG polyHIPEs were obtained with a compressive modulus of ∼2.6 MPa. Furthermore, polyHIPEs with a bilayered design could be prepared by layering emulsions consisting of all PBLG and PCL to form a single monolith with a soft top and stiff bottom with a mechanically robust interface due to the thiol–ene click reactions occurring at the interface of both emulsions.
Figure 15.
(a) Synthetic design for the preparation of PU/PE polyHIPEs with a copolymer or bilayered network. (b) Digital image of a bilayered polyHIPE (left) and digital images of the polyHIPE under elongation to highlight the difference in elasticity for each layer. Reprinted with permission from ref (95). Copyright 2022 American Chemical Society.
The direct ring-opening polymerization of ε-caprolactone (CL) in oil-in-oil emulsions has been used to synthesize entirely PCL-based polyHIPEs.96 Recently, Pahovnik, Kovačič, and co-workers97 prepared shape memory polyHIPEs with high porosity (>80%) made entirely from PCL or PCL derivatives through a direct polymerization of an oil-in-oil emulsion consisting of CL and a bis-lactone cross-linker at low temperatures. The crystallization temperature (Tc) and melting temperature (Tm) of the polyHIPEs prepared in this work were dependent on the bis-lactone cross-linker and could be used to tune the shape memory properties. Specifically, polyHIPEs with 7.5 wt % cross-linker had Tc and Tm values of ∼−20 and 10 °C, respectively, compared to polyHIPEs prepared with 5 wt % cross-linker, which had Tc and Tm values of ∼4 and 32 °C, respectively, while both formulations had shape fixity and recovery ratios of over 90% over five compression–recovery cycles.
Direct ring-opening polymerizations of CL to form polyHIPEs requires the use of oil-in-oil emulsions, and such emulsions are inherently difficult to prepare and can require large amounts of surfactants to form stable emulsions. The use of water-tolerant click reactions, such as thiol–alkene reactions, has been a way to prepare fully degradable ester-based polyHIPEs using the direct polymerization route from water-in-oil emulsions.98 For example, Krajnc and co-workers99 prepared polyHIPEs with an ester functionality from thiol–alkene click reactions between divinyl adipate (DVA) and tetrakis mercaptopropionate (TT) to be used as monolithic cartilage tissue scaffolds. The DVA/TT polyHIPE obtained a Young’s modulus (∼0.2 MPa) comparable to native cartilage tissue after 20 days of cell growth and showed significantly higher collagen II cell expression than the control monolayer scaffold. Although these polyHIPEs are not true polyesters, they have similar degradability and mechanical properties of polyHIPEs made from polymers with an ester backbone functionality. Similarly, the step-growth polymerization of small-molecule thiol–ene reactions can be used as a technique to both prepare the polymer network and perform postpolymerization surface modifications. The Cameron group has pioneered multiple thiol–ene-based polyHIPE platforms consisting of polymer networks made from multithiols such as trimethylolpropane tris(3-mercaptopropionate) (TMPTMP) and multiacrylates such as dipentaerythritol penta-/hexaacrylate (DPEHA)100−102 In those examples, the polyHIPEs are first prepared and then subsequently surface modified with thiol- or alkene-containing substrates such as maleimide-functionalized peptide sequences, resulting in enhanced biocompatibility of the scaffold. Specifically, TMPTMP/DPEHA polyHIPEs could prepared with a porosity of ∼85% and pore sizes ranging from 10 to 130 μm, and then the polyHIPE was functionalized with a maleimide-Jagged-1 peptide sequence.102 The polyHIPEs were compressible before and after functionalization, obtaining Young’s moduli of ∼15 and ∼45 kPa respectively, and the functionalized scaffolds were suitable for hematopoietic stem and progenitor cell culture ex vivo.
Alternatively, an extrusion-based 3D-printing method of water-in-oil emulsions was recently used to prepare micro- and macroporous PCL tissue scaffolds using a solvent casting approach. Srivastava and co-workers103 prepared water-in-oil Pickering emulsions using hydrophobically modified nanoclay (Cloisite 30B) as the emulsifier and PCL (Mn = 43000 g/mol) in the continues phase, and the emulsions could be extruded and then dried, without the loss of the desired pore structure due to the highly stable nature of the emulsion. The 3D-printed PCL-polyHIPEs were elastic and could be compressed to 60% without fracture, and the polyHIPEs with the highest loading content of Cloisite 30B obtained the highest Young’s modulus of ∼1.1 MPa at 2 wt % compared to ∼0.2 MPa at 1 wt %.
4.2. Flexible Electronics and Separation Membranes
Polyurethane-based polyHIPEs can be modified to obtain conductive materials by introducing electrically active additives such as carbon nanotubes. For example, Guo and co-workers104 prepared pressure-sensitive polyHIPEs by embedding acidified multiwalled carbon nanotubes (MWCNTs) within a toluene diissocyanate (TDI)/castor oil polyurethane network. A hydroxy-terminated surfactant (polyglycerol polyricinoleate) was used in high amounts (∼20 wt %) to limit unwanted side reactions between water from the dispersed phase and TDI by acting as a barrier and as an initiator for the polymerization. The conductive resistance of the polyHIPE sensor was dependent on the loading content of MWCNTs, with a minimum resistance of ∼2 MΩ being observed at 2 wt % and a maximum resistance of ∼500 MΩ seen at 0.75 wt %.
PU-based polyHIPEs have been used as components in flexible electronics and energy harvesting devices. Specifically, PU-based polyHIPEs that can undergo multiple mechanical deformation–recovery cycles can be used in capacitive energy-harvesting devices. For example, Bismarck and co-workers105 synthesized polyHIPEs with a copolymer network of commercially available polyurethane diacrylate (Ebecryl 8402) and EHA as a mechanically resilient “spring” in reverse electrowetting on dielectric energy-harvesting devices. The PU/EHA polyMIPEs and polyHIPEs were prepared with two different porosities (∼65% or 75%) to obtain springs that compressed to different degrees under the walking conditions for an average person. PolyMIPEs were more rigid and obtained an elastic modulus of ∼0.6 MPa, compared to ∼0.3 MPa for the polyHIPEs with a higher porosity. The polyHIPEs with a porosity of 75% were able to obtain a maximum capacitance change of ∼600 pF (after compressing to a load of 0.1 MPa and then releasing) when manufactured as an energy-harvesting device using mercury as the electrowetting substance.
The flexibility and interconnected pore morphology of PU-polyHIPEs can be used to prepare separation membranes in applications such as oil remediation or flexible batteries.106 For example, Kaltenbrunner and co-workers107 showed that a PU-based polyHIPE could be used as an ionic separation membrane in a sandwich-designed zinc–carbon battery to power a wearable Bluetooth controller for a speaker (Figure 16). The performance of the battery was evaluated over strained (∼20%) and relaxed states, and the battery showed no significant differences in the voltage–current profile when a PU-polyHIPE with a porosity of 85% was used as the separation membrane. Interestingly, the polyHIPEs prepared were directly templated from aqueous electrolyte (ZnCl2/NH4Cl)-in-monomer emulsions to directly imbibe the electrolyte solution, limiting the number of preparation steps required for device preparation.
Figure 16.
(left) Digital image of a battery using a polyurethane-based polyHIPE as the separation membrane being stretched and (right) digital image of a wearable Bluetooth controller powered by the flexible battery. Reprinted with permission from ref (107). Copyright 2020.
Emulsion templating of PEs and PUs is an efficient and simple method to prepare porous elastomers for applications ranging from biomaterials to wearable electronics. Continued growth in the types of ester-containing polyHIPE networks beyond PCL can be expected for tissue-engineering applications. One persistent challenge associated with PE and PU polymerizations is the intolerance to water, the most used polar phase in emulsion templating. Multistep (or prepolymerization) routes have been a way to avoid this issue, but this method introduces the need for additional comonomers or cross-linkers to be added to prepare polyHIPEs. It can be expected that the types of cross-linking chemistry, such as click reactions, will continue to be expanded to provide more aqueous-friendly routes to the synthesis of PEs and PUs.
5. Naturally Occurring Polymers
Naturally occurring polymers can be nontoxic and biodegradable and are commonly used to prepare hydrogels for biological applications such as tissue engineering and drug delivery.108 Porous hydrogels, which are hydrogels with macroscopic pores (diameter >50 nm), show enhanced water uptake, stimuli response, and flexibility compared to nonporous, or standard, hydrogels.109,110 Emulsion templating is a simple route to prepare porous hydrogels, where the template typically consists of a nonpolar dispersed phase stabilized within an aqueous continuous phase containing polymerizable monomers and cross-linkers. Elastic polyHIPE hydrogels made from naturally occurring proteins or polysaccharides such as gelatin,111−113 alginate,114 and chitosan115 have been prepared as cell culture scaffolds. In most cases, natural polymers require functionalization to a (meth)acrylate group to be compatible in the synthesis of 3D materials. For example, Ren, Han, and co-workers116 synthesized surfactant-free polyHIPE-hydrogel scaffolds for corneal stromal regeneration from two-phase aqueous emulsions of methacrylated gelatin and poly(ethylene oxide) containing solutions as the continuous phase and dispersed phase, respectively. The polyHIPE hydrogels presented in that work showed higher numbers of total cells and number of elongated cells in 5 day in vitro studies when compared to nonporous controls. Additionally, the water content for the polyHIPE hydrogels was consistent with native human cornea, but the materials had a much lower compressive Young’s modulus than native cornea, obtaining values of ∼7 kPa for the polyHIPE hydrogels compared to ∼30–40 kPa for native cornea. In related work, Zhang and co-workers111 used a similar two-phase aqueous emulsion system as bioinks for additive manufactured polyHIPE hydrogel cell culture scaffolds that showed significantly improved cell proliferation compared to traditional hydrogels (Figure 17).
Figure 17.
(left) Cartoon overview of the 3D-printing process to prepare cell-laden porous (top left) and nonporous hydrogel scaffolds from an emulsion bioink. (right) Fluorescence micrographs comparing cell proliferation in polyHIPE hydrogels to that in nonporous hydrogels. Reprinted with permission from ref (111). Copyright 2018.
Biomaterials derived from naturally occurring polymers can suffer from low mechanical properties, limiting their use in many biological systems, and to overcome this, synthetic polymers can be added as copolymers to improve their durability. For example, synthetic polymers including polyethylene glycol (PEG) and poly(N-isopropylacrylamide) (polyNIPAM) have been copolymerized with methacrylated gelatin (GelMa). Specifically, Nikfarjam and co-workers117 showed that the mechanical properties of a GelMa-based polyHIPE-hydrogel could be improved using acrylate-functionalized PEG and PCL in a semi-interpenetrating network (semi-IPN) to mimic thin skin tissue. Qualitative analysis was used to show that mechanically robust polyHIPE hydrogels could only be achieved using a combination of PEG and PCL (12 and 18 wt %, respectively) while materials with only GelMa were not capable of being handled.
Alternatively, using synthetic polymers to target the structure and properties of natural polymers has shown to be promising in improving the mechanical properties of polyHIPE hydrogel biomaterials. For example, Wynne and co-workers118 synthesized polyHIPE hydrogels that had a similar structure to alginate using a copolymer network of sodium acylate, calcium diacrylate, PEG-diacrylate, and polyNIPAM as a hemostatic wound dressing. The polyHIPEs prepared with polyNIPAM showed improved storage moduli under tension in the dry state, with polyHIPEs having 30 wt % polyNIPAM obtaining values of ∼40 MPa and materials without polyNIPAM obtaining values of ∼27 MPa. Additionally, the materials showed improved whole blood trapping when polyNIPAM was added to the polymer network, and the polyHIPE hydrogel outperformed commercially available gauze.
Recently, the Pahovnik group has prepared elastic polyHIPE hydrogels from synthetic polypeptide networks using a ring-opening polymerization of N-carboxyanhydrides from a library of amino acid building blocks that undergo pH-dependent swelling in aqueous environments for stimuli-responsive biomaterials.119 For example, Pahovnik and co-workers120 prepared polyHIPE hydrogels from glutamic acid (Glu) and copolymerizations of Glu with phenylalanine (Phe) and lysine (Lys) that showed significant swelling modulations in different pH environments (Figure 18). Specifically, polyHIPE hydrogels that consisted of a poly(Glu-co-Lys) copolymer network obtained increased swelling under both acidic and basic conditions due to the polyelectrolyte effect, while polyHIPEs consisting of only poly(Glu) and poly(Glu-co-Phe) obtained increased swelling only under highly basic conditions. Additionally, the compressive Young’s modulus of the swollen materials was dependent on the pH of the imbibed solution (i.e., swelling characteristic of the material), where poly(Glu-co-Lys) polyHIPE-hydrogels swelled the least at ∼20 g/g and were the stiffest, obtaining a value of ∼120 kPa compared to the other formulations.
Figure 18.
(a) Reaction scheme and digital image showing the swelling response of a peptide-based polyHIPE hydrogel at different pHs (left) and stress–strain curves for materials with different network compositions (right). Inset: digital image (top right) of a representative compression cycle showing good recovery. (b) Buffer uptake of the polyHIPE hydrogels at different pHs for poly(Glu) (left), poly(Glu-co-Phe) (middle), and poly(Glu-co-Lys) (right). Reprinted with permission from ref (120). Copyright 2021 American Chemical Society.
PolyHIPE hydrogels can be used as a delivery system for therapeutics when the internal phase is a pharmaceutical itself.121 For example, Rotello and co-workers122 prepared biodegradable protein-based polyHIPEs from a one-step approach where various nonpolar essential oils were used as an antimicrobial internal phase. In that work, bovine serum albumin was used as the stabilizer for the emulsion and as the monomer with dithiothreitol as the cross-linker to prepare a fully degradable network when exposed to trypsin. Furthermore, upon degradation, the polyHIPE hydrogels that released eugenol had antimicrobial properties to MRSA and E. coli, showing negligible bacterial growth surrounding the polyHIPE hydrogel after being incubated in a Petri dish for 24 h.
PolyHIPE hydrogels prepared from natural polymers like gelatin and alginate have been successful in preparing scaffolds for soft tissue engineering. One persistent challenge in naturally derived polyHIPE hydrogels is their limited mechanical properties. It can be expected that continued advances in copolymer networks of natural and biocompatible synthetic copolymers will continue to be realized, including using double and interpenetrating network approaches. Emulsions that are compatible with extrusion and stereolithographic additive manufacturing techniques will need to be realized to provide a platform for more custom synthesis of these types of porous materials.
6. Conclusions
Emulsion templating of elastomeric polymer networks is a powerful strategy for preparing PFs with a range of controllable chemical and material properties. For example, elastic polyHIPEs have been achieved with pore diameters ranging from nano- to micrometer lengths with open-cell or closed-cell pore morphologies. Recently, with the growth of microfluidics, polyHIPEs with monodisperse pore sizes can now be realized. Furthermore, the total porosity of polyHIPEs can be controlled by simply adding different amounts of a dispersed phase in the emulsion before polymerization. This comprehensive control over the porous nature of elastic polyHIPEs has been a key factor in the development of polyHIPEs that are used in applications including tissue engineering, pressure sensors, and recyclable separation membranes, where controlling the pore size has improved the performance of the materials.
The polyHIPE method is compatible with many polymerization conditions including aqueous, organic, bulk, and moisture or oxygen sensitivity, allowing for the translation of many polymerization processes to the polyHIPE field. For example, the adaptation of rapid photoinitiated radical polymerizations to emulsion templating has made the realization of elastic PF with homogeneous pore morphologies from deposition-based additive manufacturing techniques possible, a challenge that has been outlined in additive manufacturing.123 The adaptation of the state-of-the-art materials and polymer synthesis methods to polyHIPEs can be expected to continue.
Elastic polyHIPEs meet the requirements of many emerging technologies, including wearable electronics and soft robotics that rely on integration of multiple functionalities into a single elastomer. For example, the development of elastic polyHIPE composites that have conductive components have been successful in the evolution of novel sensors and energy storage devices.105,107 Emulsions that are compatible with extrusion and stereolithographic additive manufacturing techniques will continue to need to be realized to provide platforms for new custom syntheses of these porous materials, and flexible polyHIPEs with multiple available reactive functional groups will continue to be developed and applied to diverse application areas.
Acknowledgments
We thank the National Science Foundation (DMR-1940518) for the resources to conduct this work.
The authors declare no competing financial interest.
References
- Ayushi C.; Sagnik C.; Eswara P.. Polymer Foam Market; Portland, OR, 2020. [Google Scholar]
- Polymer Foam Market Research Report; New York, NY, 2023. [Google Scholar]
- Gama N. v.; Ferreira A.; Barros-Timmons A.. Polyurethane Foams: Past, Present, and Future. Materials. MDPI: AG, September 27, 2018. 10.3390/ma11101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin F. L.; Zhao M.; Park M.; Park S. J. Recent Trends of Foaming in Polymer Processing: A Review. Polymers (Basel) 2019, 11 (6), 953. 10.3390/polym11060953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels H. W.; Pirkl H. G.; Albers R.; Albach R. W.; Krause J.; Hoffmann A.; Casselmann H.; Dormish J. Polyurethanes: Versatile Materials and Sustainable Problem Solvers for Today’s Challenges. Angewandte Chemie - International Edition. 2013, 52, 9422–9441. September 2 10.1002/anie.201302766. [DOI] [PubMed] [Google Scholar]
- Altan M.Thermoplastic Foams: Processing, Manufacturing, and Characterization. Recent Research in Polymerization; InTechOpen: 2018. 10.5772/intechopen.71083 [DOI] [Google Scholar]
- Shastri V. P.; Martin I.; Langer R. Macroporous Polymer Foams by Hydrocarbon Templating. Proc. Natl. Acad. Sci. U. S. A. 2000, 97 (5), 1970–1975. 10.1073/pnas.97.5.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau M. R.; Grunlan M. A. Smart Scaffolds: Shape Memory Polymers (SMPs) in Tissue Engineering. J. Mater. Chem. B 2021, 9 (21), 4287–4297. 10.1039/D1TB00607J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton E.; Chai Q.; Shaw M. W.; McKenzie T. J.; Mullins E. S.; Ayres N. Hydrogel-Coated Polyurethane/Urea Shape Memory Polymer Foams. J. Polym. Sci. A Polym. Chem. 2019, 57 (13), 1389–1395. 10.1002/pola.29398. [DOI] [Google Scholar]
- Wake M. C.; Gupta P. K.; Mikos A. G. Fabrication of Pliable Biodegradable Polymer Foams to Engineer Soft Tissues. Cell Transplant 1996, 5 (4), 465–473. 10.1016/0963-6897(96)00025-5. [DOI] [PubMed] [Google Scholar]
- Bartl V. H.; von Bonin W. Über Die Polymerisation in Umgekehrter Emulsion. II. Makromol. Chem. 1963, 66 (1), 151–156. 10.1002/macp.1963.020660115. [DOI] [Google Scholar]
- Haq Z.; Barby D.. Low Density Porous Cross-Linked Polymeric Materials and Their Preparation. EP0060138A1, 1982.
- Dabrowski M. L.; Jenkins D.; Cosgriff-Hernandez E.; Stubenrauch C. Methacrylate-Based Polymer Foams with Controllable Connectivity, Pore Shape, Pore Size and Polydispersity. Phys. Chem. Chem. Phys. 2020, 22 (1), 155–168. 10.1039/C9CP03606G. [DOI] [PubMed] [Google Scholar]
- Foudazi R. HIPEs to PolyHIPEs. React. Funct Polym. 2021, 164 (April), 104917. 10.1016/j.reactfunctpolym.2021.104917. [DOI] [Google Scholar]
- Zhang T.; Sanguramath R. A.; Israel S.; Silverstein M. S. Emulsion Templating: Porous Polymers and Beyond. Macromolecules 2019, 52 (15), 5445–5479. 10.1021/acs.macromol.8b02576. [DOI] [Google Scholar]
- Cameron N. R.; Sherrington D. C.; Albiston L.; Gregory D. P. Study of the Formation of the Open-Cellular Morphology of Poly(Styrene/Divinylbenzene) PolyHIPE Materials by Cryo-SEM. Colloid Polym. Sci. 1996, 274 (6), 592–595. 10.1007/BF00655236. [DOI] [Google Scholar]
- Hainey P.; Huxham I. M.; Rowatt B.; Sherrington D. C.; Tetley L. Synthesis and Ultrastructural Studies, of Styrene-Divinylbenzene Polyhipe Polymers. Macromolecules 1991, 24 (1), 117–121. 10.1021/ma00001a019. [DOI] [Google Scholar]
- Tai H.; Sergienko A.; Silverstein M. S. Organic-Inorganic Networks in Foams from High Internal Phase Emulsion Polymerizations. Polymer (Guildf) 2001, 42 (10), 4473–4482. 10.1016/S0032-3861(00)00820-X. [DOI] [Google Scholar]
- Dizge N.; Keskinler B.; Tanriseven A. Biodiesel Production from Canola Oil by Using Lipase Immobilized onto Hydrophobic Microporous Styrene-Divinylbenzene Copolymer. Biochem Eng. J. 2009, 44 (2–3), 220–225. 10.1016/j.bej.2008.12.008. [DOI] [Google Scholar]
- Pulko I.; Kolar M.; Krajnc P. Atrazine Removal by Covalent Bonding to Piperazine Functionalized PolyHIPEs. Sci. Total Environ. 2007, 386 (1–3), 114–123. 10.1016/j.scitotenv.2007.06.032. [DOI] [PubMed] [Google Scholar]
- Ye Y.; Jin M.; Wan D. One-Pot Synthesis of Porous Monolith-Supported Gold Nanoparticles as an Effective Recyclable Catalyst. J. Mater. Chem. A Mater. 2015, 3 (25), 13519–13525. 10.1039/C5TA02925B. [DOI] [Google Scholar]
- Aldemir Dikici B.; Claeyssens F. Basic Principles of Emulsion Templating and Its Use as an Emerging Manufacturing Method of Tissue Engineering Scaffolds. Front Bioeng Biotechnol 2020, 8, 1. 10.3389/fbioe.2020.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y.; Zhao J.; Johnson J. A. Polymer Networks: From Plastics and Gels to Porous Frameworks. Angewandte Chemie - International Edition 2020, 59 (13), 5022–5049. 10.1002/anie.201902900. [DOI] [PubMed] [Google Scholar]
- Abdou-Sabet S.; Puydak R. C.; Rader C. P. Dynamically Vulcanized Thermoplastic Elastomers. Rubber Chem. Technol. 1996, 69 (3), 476–494. 10.5254/1.3538382. [DOI] [Google Scholar]
- Cameron N. R.; Sherrington D. C. Preparation and Glass Transition Temperatures of Elastomeric PolyHIPE Materials. J. Mater. Chem. 1997, 7 (11), 2209–2212. 10.1039/a702030i. [DOI] [Google Scholar]
- Tunc Y.; Hasirci N.; Ulubayram K. Synthesis of Emulsion-Templated Acrylic-Based Porous Polymers: From Brittle to Elastomeric. Soft Mater. 2012, 10 (4), 449–461. 10.1080/1539445X.2010.532848. [DOI] [Google Scholar]
- Horowitz R.; Lamson M.; Cohen O.; Fu T. B.; Cuthbert J.; Matyjaszewski K.; Silverstein M. S. Highly Efficient and Tunable Miktoarm Stars for HIPE Stabilization and PolyHIPE Synthesis. Polymer (Guildf) 2021, 217, 123444. 10.1016/j.polymer.2021.123444. [DOI] [Google Scholar]
- Gurevitch I.; Silverstein M. S. Shape Memory Polymer Foams from Emulsion Templating. Soft Matter 2012, 8 (40), 10378–10387. 10.1039/c2sm26404h. [DOI] [Google Scholar]
- Hori K.; Sano M.; Suzuki M.; Hanabusa K. Preparation of Porous Polymer Materials Using Water-in-Oil Gel Emulsions as Templates. Polym. Int. 2018, 67 (7), 909–916. 10.1002/pi.5579. [DOI] [Google Scholar]
- Normatov J.; Silverstein M. S. Highly Porous Elastomer-Silsesquioxane Nanocomposites Synthesized Within High Internal Phase Emulsions. J. Polym. Sci. A Polym. Chem. 2008, 46, 2357–2366. 10.1002/pola.22570. [DOI] [Google Scholar]
- Guan X.; Jiang H.; Ngai T. Pickering High Internal Phase Emulsions Templated Super-Hydrophobic - Oleophilic Elastic Foams for Highly Efficient Oil/Water Separation. ACS Appl. Polym. Mater. 2020, 2 (12), 5664–5673. 10.1021/acsapm.0c00976. [DOI] [Google Scholar]
- Mert E. H.; Kekevi B. Synthesis of PolyHIPEs through High Internal Phase Emulsions of β-Myrcene. Colloid Polym. Sci. 2020, 298 (10), 1423–1432. 10.1007/s00396-020-04730-4. [DOI] [Google Scholar]
- Pahovnik D.; Majer J.; Žagar E.; Kovačič S. Synthesis of Hydrogel PolyHIPEs from Functionalized Glycidyl Methacrylate. Polym. Chem. 2016, 7 (32), 5132–5138. 10.1039/C6PY01122E. [DOI] [Google Scholar]
- Jerenec S.; Šimić M.; Savnik A.; Podgornik A.; Kolar M.; Turnšek M.; Krajnc P. Glycidyl Methacrylate and Ethylhexyl Acrylate Based PolyHIPE Monoliths: Morphological, Mechanical and Chromatographic Properties. React. Funct Polym. 2014, 78 (1), 32–37. 10.1016/j.reactfunctpolym.2014.02.011. [DOI] [Google Scholar]
- Kramer S.; Cameron N. R.; Krajnc P. Porous Polymers from High Internal Phase Emulsions as Scaffolds for Biological Applications. Polymers (Basel) 2021, 13 (11), 1786. 10.3390/polym13111786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moglia R. S.; Holm J. L.; Sears N. A.; Wilson C. J.; Harrison D. M.; Cosgriff-Hernandez E. Injectable PolyHIPEs as High-Porosity Bone Grafts. Biomacromolecules 2011, 12 (10), 3621–3628. 10.1021/bm2008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moglia R. S.; Whitely M.; Dhavalikar P.; Robinson J.; Pearce H.; Brooks M.; Stuebben M.; Cordner N.; Cosgriff-Hernandez E. Injectable Polymerized High Internal Phase Emulsions with Rapid in Situ Curing. Biomacromolecules 2014, 15 (8), 2870–2878. 10.1021/bm500754r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. L.; Moglia R. S.; Stuebben M. C.; Mcenery M. A. P.; Cosgriff-Hernandez E. Achieving Interconnected Pore Architecture in Injectable PolyHIPEs for Bone Tissue Engineering. Tissue Eng. Part A 2014, 20 (5–6), 1103–1112. 10.1089/ten.tea.2013.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R.; Sherborne C.; Paterson T.; Green N. H.; Reilly G. C.; Claeyssens F. Emulsion Templated Scaffolds with Tunable Mechanical Properties for Bone Tissue Engineering. J. Mech Behav Biomed Mater. 2016, 54, 159–172. 10.1016/j.jmbbm.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. W.; Sherborne C.; Didsbury M. P.; Pateman C.; Cameron N. R.; Claeyssens F. Macrostructuring of Emulsion-Templated Porous Polymers by 3D Laser Patterning. Adv. Mater. 2013, 25, 3178. 10.1002/adma.201300552. [DOI] [PubMed] [Google Scholar]
- Sears N. A.; Dhavalikar P. S.; Cosgriff-hernandez E. M. Emulsion Inks for 3D Printing of High Porosity Materials. Macromol. Rapid Commun. 2016, 37, 1369–1374. 10.1002/marc.201600236. [DOI] [PubMed] [Google Scholar]
- Whitely M.; Cereceres S.; Dhavalikar P.; Salhadar K.; Wilems T.; Smith B.; Mikos A.; Cosgriff-Hernandez E. Improved in Situ Seeding of 3D Printed Scaffolds Using Cell-Releasing Hydrogels. Biomaterials 2018, 185, 194–204. 10.1016/j.biomaterials.2018.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherborne C.; Claeyssens F. Considerations Using Additive Manufacture of Emulsion Inks to Produce Respiratory Protective Filters Against Viral Respiratory Tract Infections Such as the COVID-19 Virus. Int. J. Bioprint 2020, 7 (1), 47–65. 10.18063/ijb.v7i1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister S. J. Porous Scaffold Design for Tissue Engineering. Nature Mater 2005, 4, 518. 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- Annabi N.; Nichol J. W.; Zhong X.; Ji C.; Koshy S.; Khademhosseini A.; Dehghani F. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16 (4), 371–383. 10.1089/ten.teb.2009.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalawade A. C.; Ghorpade R. v.; Shadbar S.; Qureshi M. S.; Chavan N. N.; Khan A. A.; Ponrathnam S. Inverse High Internal Phase Emulsion Polymerization (i-HIPE) of GMMA, HEMA and GDMA for the Preparation of Superporous Hydrogels as a Tissue Engineering Scaffold. J. Mater. Chem. B 2016, 4 (3), 450–460. 10.1039/C5TB01873K. [DOI] [PubMed] [Google Scholar]
- Cohen N.; Silverstein M. S. One-Pot Emulsion-Templated Synthesis of an Elastomer-Filled Hydrogel Framework. Macromolecules 2012, 45 (3), 1612–1621. 10.1021/ma2027337. [DOI] [Google Scholar]
- Kovačič J. M.; Ciringer T.; Ambrožič-Dolinšek J.; Kovačič S. Use of Emulsion-Templated, Highly Porous Polyelectrolytes for In Vitro Germination of Chickpea Embryos: A New Substrate for Soilless Cultivation. Biomacromolecules 2022, 23 (8), 3452–3457. 10.1021/acs.biomac.2c00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahovnik D.; Majer J.; Žagar E.; Kovačič S. Synthesis of Hydrogel PolyHIPEs from Functionalized Glycidyl Methacrylate. Polym. Chem. 2016, 7 (32), 5132–5138. 10.1039/C6PY01122E. [DOI] [Google Scholar]
- Kovačič S.; Silverstein M. S. Superabsorbent, High Porosity, PAMPS-Based Hydrogels through Emulsion Templating. Macromol. Rapid Commun. 2016, 37 (22), 1814–1819. 10.1002/marc.201600249. [DOI] [PubMed] [Google Scholar]
- Kovačič S.; Drašinac N.; Pintar A.; Žagar E. Highly Porous Cationic Polyelectrolytes via Oil-in-Water Concentrated Emulsions: Synthesis and Adsorption Kinetic Study. Langmuir 2018, 34 (35), 10353–10362. 10.1021/acs.langmuir.8b01645. [DOI] [PubMed] [Google Scholar]
- Jurjevec S.; Žagar E.; Kovačič S. Functional Macroporous Amphoteric Polyelectrolyte Monoliths with Tunable Structures and Properties through Emulsion-Templated Synthesis. J. Colloid Interface Sci. 2020, 575, 480–488. 10.1016/j.jcis.2020.05.016. [DOI] [PubMed] [Google Scholar]
- Métivier T.; Cassagnau P. New Trends in Cellular Silicone: Innovations and Applications. Journal of Cellular Plastics. 2019, 55, 151–200. 10.1177/0021955X18806845. [DOI] [Google Scholar]
- Grosse M.-T.; Lamotte M.; Birot M.; Deleuze H. Preparation of Microcellular Polysiloxane Monoliths. J. Polym. Sci. A Polym. Chem. 2008, 46 (1), 21–32. 10.1002/pola.22351. [DOI] [Google Scholar]
- Dufaud O.; Favre E.; Sadtler V. Porous Elastomeric Beads from Crosslinked Emulsions. J. Appl. Polym. Sci. 2002, 83 (5), 967–971. 10.1002/app.2276. [DOI] [Google Scholar]
- Oh M. J.; Ryu T. K.; Choi S. W. Hollow Polydimethylsiloxane Beads with a Porous Structure for Cell Encapsulation. Macromol. Rapid Commun. 2013, 34 (21), 1728–1733. 10.1002/marc.201300669. [DOI] [PubMed] [Google Scholar]
- Davis A.; Surdo S.; Caputo G.; Bayer I. S.; Athanassiou A. Environmentally Benign Production of Stretchable and Robust Superhydrophobic Silicone Monoliths. ACS Appl. Mater. Interfaces 2018, 10 (3), 2907–2917. 10.1021/acsami.7b15088. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Zhang Y.; Chen P.; Du W.; Feng X.; Liu B. F. Paraffin Oil Based Soft-Template Approach to Fabricate Reusable Porous PDMS Sponge for Effective Oil/Water Separation. Langmuir 2019, 35 (34), 11123–11131. 10.1021/acs.langmuir.9b01861. [DOI] [PubMed] [Google Scholar]
- Tu S.; Chen M.; Wu L. Robust Porous Organosilica Monoliths via a Surfactant-Free High Internal Phase Emulsion Process for Efficient Oil-Water Separation. J. Colloid Interface Sci. 2020, 566, 338–346. 10.1016/j.jcis.2020.01.053. [DOI] [PubMed] [Google Scholar]
- Vasquez L.; Davis A.; Gatto F.; Ngoc An M.; Drago F.; Pompa P. P.; Athanassiou A.; Fragouli D. Multifunctional PDMS PolyHIPE Filters for Oil-Water Separation and Antibacterial Activity. Separation and Purificiation Technology 2021, 255, 117748. 10.1016/j.seppur.2020.117748. [DOI] [Google Scholar]
- Vilanova N.; Kolen’ko Y. v.; Solans C.; Rodríguez-Abreu C. Multiple Emulsions as Soft Templates for the Synthesis of Multifunctional Silicone Porous Particles. J. Colloid Interface Sci. 2015, 437, 235–243. 10.1016/j.jcis.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Lim S. J.; Lim H. S.; Joo Y.; Jeon D. Y. Impact of MWCNT Concentration on the Piezo-Impedance Response of Porous MWCNT/PDMS Composites. Sens Actuators A Phys. 2020, 315, 112332. 10.1016/j.sna.2020.112332. [DOI] [Google Scholar]
- Xu J.; Li H.; Yin Y.; Li X.; Cao J.; Feng H.; Bao W.; Tan H.; Xiao F.; Zhu G. High Sensitivity and Broad Linearity Range Pressure Sensor Based on Hierarchical In-Situ Filling Porous Structure. npj Flexible Electronics 2022, 6 (1), 62. 10.1038/s41528-022-00191-7. [DOI] [Google Scholar]
- Kurup L. A.; Arthur J. N.; Yambem S. D. Highly Sensitive Capacitive Low-Pressure Graphene Porous Foam Sensors. ACS Appl. Electron Mater. 2022, 4 (8), 3962–3972. 10.1021/acsaelm.2c00616. [DOI] [Google Scholar]
- Oh J.; Kim J.; Kim Y.; Choi H. B.; Yang J. C.; Lee S.; Pyatykh M.; Kim J.; Sim J. Y.; Park S. Highly Uniform and Low Hysteresis Piezoresistive Pressure Sensors Based on Chemical Grafting of Polypyrrole on Elastomer Template with Uniform Pore Size. Small 2019, 15 (33), 1901744. 10.1002/smll.201901744. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Yang H.; Oh J. H. Simple Fabrication of Highly Sensitive Capacitive Pressure Sensors Using a Porous Dielectric Layer with Cone-Shaped Patterns. Mater. Des 2021, 197, 109203. 10.1016/j.matdes.2020.109203. [DOI] [Google Scholar]
- Yang C. R.; Wang L. J.; Tseng S. F. Arrayed Porous Polydimethylsiloxane/Barium Titanate Microstructures for High-Sensitivity Flexible Capacitive Pressure Sensors. Ceram. Int. 2022, 48 (9), 13144–13153. 10.1016/j.ceramint.2022.01.191. [DOI] [Google Scholar]
- Hwang J.; Kim Y.; Yang H.; Oh J. H. Fabrication of Hierarchically Porous Structured PDMS Composites and Their Application as a Flexible Capacitive Pressure Sensor. Compos B Eng. 2021, 211, 108607. 10.1016/j.compositesb.2021.108607. [DOI] [Google Scholar]
- Zimny K.; Merlin A.; Ba A.; Aristégui C.; Brunet T.; Mondain-Monval O. Soft Porous Silicone Rubbers as Key Elements for the Realization of Acoustic Metamaterials. Langmuir 2015, 31 (10), 3215–3221. 10.1021/la504720f. [DOI] [PubMed] [Google Scholar]
- Kovalenko A.; Zimny K.; Mascaro B.; Brunet T.; Mondain-Monval O. Tailoring of the Porous Structure of Soft Emulsion-Templated Polymer Materials. Soft Matter 2016, 12 (23), 5154–5163. 10.1039/C6SM00461J. [DOI] [PubMed] [Google Scholar]
- Kovalenko A.; Fauquignon M.; Brunet T.; Mondain-Monval O. Tuning the Sound Speed in Macroporous Polymers with a Hard or Soft Matrix. Soft Matter 2017, 13 (25), 4526–4532. 10.1039/C7SM00744B. [DOI] [PubMed] [Google Scholar]
- Brunet T.; Merlin A.; Mascaro B.; Zimny K.; Leng J.; Poncelet O.; Aristégui C.; Mondain-Monval O. Soft 3D Acoustic Metamaterial with Negative Index. Nat. Mater. 2015, 14 (4), 384–388. 10.1038/nmat4164. [DOI] [PubMed] [Google Scholar]
- Jin Y.; Kumar R.; Poncelet O.; Mondain-Monval O.; Brunet T. Flat Acoustics with Soft Gradient-Index Metasurfaces. Nat. Commun. 2019, 10 (1), 143. 10.1038/s41467-018-07990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie T. J.; Heaton P. S.; Rishi K.; Kumar R.; Brunet T.; Beaucage G.; Mondain-Monval O.; Ayres N. Storage Moduli and Porosity of Soft PDMS PolyMIPEs Can. Be Controlled Independently Using Thiol-Ene Click Chemistry. Macromolecules 2020, 53 (10), 3719–3727. 10.1021/acs.macromol.0c00217. [DOI] [Google Scholar]
- McKenzie T. J.; Smail S.; Rost K.; Rishi K.; Beaucage G.; Ayres N. Multi-Layered Polymerized High Internal Phase Emulsions with Controllable Porosity and Strong Interfaces. Polymer (Guildf) 2021, 231, 124116. 10.1016/j.polymer.2021.124116. [DOI] [Google Scholar]
- McKenzie T. J.; Rost K.; Smail S.; Mondain-Monval O.; Brunet T.; Ayres N. Mechanically Tunable PDMS-Based PolyHIPE Acoustic Materials. J. Mater. Chem. C Mater. 2022, 10 (16), 6222–6226. 10.1039/D2TC00136E. [DOI] [Google Scholar]
- Tebboth M.; Jiang Q.; Kogelbauer A.; Bismarck A. Inflatable Elastomeric Macroporous Polymers Synthesized from Medium Internal Phase Emulsion Templates. ACS Appl. Mater. Interfaces 2015, 7, 19243–19250. 10.1021/acsami.5b05123. [DOI] [PubMed] [Google Scholar]
- Bury E.; Thiagarajan S.; Lazarus N.; Koh A. Ferrofluid High Internal Phase Emulsion Polymer Foams for Soft, Magnetic Materials. J. Magn Magn Mater. 2022, 563, 169921. 10.1016/j.jmmm.2022.169921. [DOI] [Google Scholar]
- Giustiniani A.; Guégan P.; Marchand M.; Poulard C.; Drenckhan W. Generation of Silicone Poly-HIPEs with Controlled Pore Sizes via Reactive Emulsion Stabilization. Macromol. Rapid Commun. 2016, 37 (18), 1527–1532. 10.1002/marc.201600281. [DOI] [PubMed] [Google Scholar]
- Kataruka A.; Hutchens S. B. PDMS Polymerized High Internal Phase Emulsions (PolyHIPEs) with Closed-Cell, Aqueous-Filled Microcavities. Soft Matter 2019, 15 (47), 9665–9675. 10.1039/C9SM01732A. [DOI] [PubMed] [Google Scholar]
- Kataruka A.; Hutchens S. B. Swelling of a Non-Vascular-Plant-Inspired Soft Composite. Matter 2021, 4 (12), 3991–4005. 10.1016/j.matt.2021.10.015. [DOI] [Google Scholar]
- Busby W.; Cameron N. R.; Jahoda C. A. B. Emulsion-Derived Foams (PolyHIPEs) Containing Poly(ε-Caprolactone) as Matrixes for Tissue Engineering. Biomacromolecules 2001, 2 (1), 154–164. 10.1021/bm0000889. [DOI] [PubMed] [Google Scholar]
- Lumelsky Y.; Lalush-Michael I.; Levenberg S.; Silverstein M. S. A Degradable, Porous, Emulsion-Templated Polyacrylate. J. Polym. Sci. A Polym. Chem. 2009, 47 (24), 7043–7053. 10.1002/pola.23744. [DOI] [Google Scholar]
- Lumelsky Y.; Silverstein M. S. Biodegradable Porous Polymers through Emulsion Templating. Macromolecules 2009, 42 (5), 1627–1633. 10.1021/ma802461m. [DOI] [Google Scholar]
- Aldemir Dikici B.; Sherborne C.; Reilly G. C.; Claeyssens F. Emulsion Templated Scaffolds Manufactured from Photocurable Polycaprolactone. Polymer (Guildf) 2019, 175, 243–254. 10.1016/j.polymer.2019.05.023. [DOI] [Google Scholar]
- Dikici S.; Aldemir Dikici B.; Macneil S.; Claeyssens F. Decellularised Extracellular Matrix Decorated PCL PolyHIPE Scaffolds for Enhanced Cellular Activity, Integration and Angiogenesis. Biomater Sci. 2021, 9 (21), 7297–7310. 10.1039/D1BM01262B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pok S.; Myers J. D.; Madihally S. v.; Jacot J. G. A Multilayered Scaffold of a Chitosan and Gelatin Hydrogel Supported by a PCL Core for Cardiac Tissue Engineering. Acta Biomater 2013, 9 (3), 5630–5642. 10.1016/j.actbio.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X.; Ji C.; Chan A. K. L.; Kazarian S. G.; Ruys A.; Dehghani F. Fabrication of Chitosan/Poly(ε-Caprolactone) Composite Hydrogels for Tissue Engineering Applications. J. Mater. Sci. Mater. Med. 2011, 22 (2), 279–288. 10.1007/s10856-010-4194-2. [DOI] [PubMed] [Google Scholar]
- Ilagan B. G.; Amsden B. G. Macroporous Photocrosslinked Elastomer Scaffolds Containing Microposity: Preparation and in Vitro Degradation Properties. J. Biomed Mater. Res. A 2009, 9999A, 211–218. 10.1002/jbm.a.32482. [DOI] [PubMed] [Google Scholar]
- Moglia R. S.; Robinson J. L.; Muschenborn A. D.; Touchet T. J.; Maitland D. J.; Cosgriff-Hernandez E. Injectable PolyMIPE Scaffolds for Soft Tissue Regeneration. Polymer (Guildf) 2014, 55 (1), 426–434. 10.1016/j.polymer.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changotade S.; Radu Bostan G.; Consalus A.; Poirier F.; Peltzer J.; Lataillade J. J.; Lutomski D.; Rohman G. Preliminary in Vitro Assessment of Stem Cell Compatibility with Cross-Linked Poly(ε -Caprolactone Urethane) Scaffolds Designed through High Internal Phase Emulsions. Stem Cells Int. 2015, 2015, 1. 10.1155/2015/283796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapilov-Buchman K.; Bialystocki T.; Niezni D.; Perry L.; Levenberg S.; Silverstein M. S. Porous Polycaprolactone and Polycarbonate Poly(Urethane Urea)s via Emulsion Templating: Structures, Properties, Cell Growth. Polym. Chem. 2021, 12 (45), 6569–6581. 10.1039/D1PY01106E. [DOI] [Google Scholar]
- Johnson D. W.; Langford C. R.; Didsbury M. P.; Lipp B.; Przyborski S. A.; Cameron N. R. Fully Biodegradable and Biocompatible Emulsion Templated Polymer Scaffolds by Thiol-Acrylate Polymerization of Polycaprolactone Macromonomers. Polym. Chem. 2015, 6 (41), 7256–7263. 10.1039/C5PY00721F. [DOI] [Google Scholar]
- Aldemir Dikici B.; Malayeri A.; Sherborne C.; Dikici S.; Paterson T.; Dew L.; Hatton P.; Ortega Asencio I.; MacNeil S.; Langford C.; Cameron N. R.; Claeyssens F. Thiolene-and Polycaprolactone Methacrylate-Based Polymerized High Internal Phase Emulsion (PolyHIPE) Scaffolds for Tissue Engineering. Biomacromolecules 2022, 23 (3), 720–730. 10.1021/acs.biomac.1c01129. [DOI] [PubMed] [Google Scholar]
- Utrosa P.; Gradisar S.; Onder O. C.; Zagar E.; Pahovnik D. Synthetic Polypeptide-Polyester PolyHIPEs Prepared by Thiol-Ene Photopolymerization. Macromolecules 2022, 55 (13), 5892–5900. 10.1021/acs.macromol.2c00926. [DOI] [Google Scholar]
- Yadav A.; Pal J.; Nandan B.; Srivastava R. K. Macroporous Scaffolds of Cross-Linked Poly(ε-Caprolactone)via High Internal Phase Emulsion Templating. Polymer (Guildf) 2019, 176, 66–73. 10.1016/j.polymer.2019.05.034. [DOI] [Google Scholar]
- Utroša P.; Onder O. C.; Žagar E.; Kovačič S.; Pahovnik D. Shape Memory Behavior of Emulsion-Templated Poly(ϵ-Caprolactone) Synthesized by Organocatalyzed Ring-Opening Polymerization. Macromolecules 2019, 52 (23), 9291–9298. 10.1021/acs.macromol.9b01780. [DOI] [Google Scholar]
- Hobiger V.; Zahoranova A.; Baudis S.; Liska R.; Krajnc P. Thiol-Ene Cross-Linking of Poly(Ethylene Glycol) within High Internal Phase Emulsions: Degradable Hydrophilic PolyHIPEs for Controlled Drug Release. Macromolecules 2021, 54 (22), 10370–10380. 10.1021/acs.macromol.1c01240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranda J.; Sušec M.; Maver U.; Gradišnik L.; Gorenjak M.; Vukasović A.; Ivković A.; Rupnik M. S.; Vogrin M.; Krajnc P. Polyester Type PolyHIPE Scaffolds with an Interconnected Porous Structure for Cartilage Regeneration. Sci. Rep 2016, 6, 28695. 10.1038/srep28695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.; Langford C. R.; Rodriguez-Lorenzo L. M.; Thissen H.; Cameron N. R. Bioceramic Nanocomposite Thiol-Acrylate PolyHIPE Scaffolds for Enhanced Osteoblastic Cell Culture in 3D. Biomater Sci. 2017, 5 (10), 2035–2047. 10.1039/C7BM00292K. [DOI] [PubMed] [Google Scholar]
- Eissa A. M.; Wilson P.; Chen C.; Collins J.; Walker M.; Haddleton D. M.; Cameron N. R. Reversible Surface Functionalisation of Emulsionlated Porous Polymers Using Dithiophenol Maleimide Functional Macromolecules. Chem. Commun. 2017, 53 (70), 9789–9792. 10.1039/C7CC03811A. [DOI] [PubMed] [Google Scholar]
- Severn C. E.; Eissa A. M.; Langford C. R.; Parker A.; Walker M.; Dobbe J. G. G.; Streekstra G. J.; Cameron N. R.; Toye A. M. Ex Vivo Culture of Adult CD34+ Stem Cells Using Functional Highly Porous Polymer Scaffolds to Establish Biomimicry of the Bone Marrow Niche. Biomaterials 2019, 225 (April), 119533. 10.1016/j.biomaterials.2019.119533. [DOI] [PubMed] [Google Scholar]
- Ghosh S.; Yadav A.; Rani S.; Takkar S.; Kulshreshtha R.; Nandan B.; Srivastava R. K. 3D Printed Hierarchical Porous Poly(ϵ-Caprolactone) Scaffolds from Pickering High Internal Phase Emulsion Templating. Langmuir 2023, 39, 1927. 10.1021/acs.langmuir.2c02936. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Du Z.; Zou W.; Li H.; Zhang C.; Li S.; Guo W. A Porous Elastomeric Polyurethane Monolith Synthesized by Concentrated Emulsion Templating and Its Pressure-Sensitive Conductive Property. RSC Adv. 2015, 5 (81), 65890–65896. 10.1039/C5RA12072A. [DOI] [Google Scholar]
- Jiang Q.; Barkan H.; Menner A.; Bismarck A. Micropatterned, Macroporous Polymer Springs for Capacitive Energy Harvesters. Polymer (Guildf) 2017, 126, 419–424. 10.1016/j.polymer.2017.04.018. [DOI] [Google Scholar]
- Zhang T.; Gui H.; Xu Z.; Zhao Y. Hydrophobic Polyurethane PolyHIPEs Templated from Mannitol within Nonaqueous High Internal Phase Emulsions for Oil Spill Recovery. J. Polym. Sci. A Polym. Chem. 2019, 57 (12), 1315–1321. 10.1002/pola.29392. [DOI] [Google Scholar]
- Danninger D.; Hartmann F.; Paschinger W.; Pruckner R.; Schwödiauer R.; Demchyshyn S.; Bismarck A.; Bauer S.; Kaltenbrunner M. Stretchable Polymerized High Internal Phase Emulsion Separators for High Performance Soft Batteries. Adv. Energy Mater. 2020, 10 (19), 2000467. 10.1002/aenm.202000467. [DOI] [Google Scholar]
- Puertas-Bartolomé M.; Mora-Boza A.; García-Fernández L. Emerging Biofabrication Techniques: A Review on Natural Polymers for Biomedical Applications. Polymers. 2021, 13, 1209. 10.3390/polym13081209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed E. M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6 (2), 105–121. 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Grady M. L.; Kuo P. L.; Parker K. K. Optimization of Electroactive Hydrogel Actuators. ACS Appl. Mater. Interfaces 2010, 2 (2), 343–346. 10.1021/am900755w. [DOI] [PubMed] [Google Scholar]
- Ying G. L.; Jiang N.; Maharjan S.; Yin Y. X.; Chai R. R.; Cao X.; Yang J. Z.; Miri A. K.; Hassan S.; Zhang Y. S. Aqueous Two-Phase Emulsion Bioink-Enabled 3D Bioprinting of Porous Hydrogels. Adv. Mater. 2018, 30 (50), 1805460. 10.1002/adma.201805460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbetta A.; Dentini M.; Zannoni E. M.; de Stefano M. E. Tailoring the Porosity and Morphology of Gelatin-Methacrylate PolyHIPE Scaffolds for Tissue Engineering Applications. Langmuir 2005, 21 (26), 12333–12341. 10.1021/la0520233. [DOI] [PubMed] [Google Scholar]
- Barbetta A.; Massimi M.; Devirgiliis L. C.; Dentini M. Enzymatic Cross-Linking versus Radical Polymerization in the Preparation of Gelatin PolyHIPEs and Their Performance as Scaffolds in the Culture of Hepatocytes. Biomacromolecules 2006, 7 (11), 3059–3068. 10.1021/bm060533l. [DOI] [PubMed] [Google Scholar]
- Barbetta A.; Barigelli E.; Dentini M. Porous Alginate Hydrogels: Synthetic Methods for Tailoring the Porous Texture. Biomacromolecules 2009, 10 (8), 2328–2337. 10.1021/bm900517q. [DOI] [PubMed] [Google Scholar]
- Tripodo G.; Calleri E.; di Franco C.; Torre M. L.; Memo M.; Mandracchia D. Inverse Poly-High Internal Phase Emulsions Poly(Hipes) Materials from Natural and Biocompatible Polysaccharides. Materials 2020, 13 (23), 5499. 10.3390/ma13235499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X.; Song W.; Sun X.; Liu J.; Huang Y.; Shen J.; Liu S.; Han Q.; Ren L. Porous Hydrogel Constructs Based on Methacrylated Gelatin/Polyethylene Oxide for Corneal Stromal Regeneration. Mater. Today Commun. 2022, 32, 104071. 10.1016/j.mtcomm.2022.104071. [DOI] [Google Scholar]
- Safaei-Yaraziz A.; Akbari-Birgani S.; Nikfarjam N. Porous Scaffolds with the Structure of an Interpenetrating Polymer Network Made by Gelatin Methacrylated Nanoparticle-Stabilized High Internal Phase Emulsion Polymerization Targeted for Tissue Engineering. RSC Adv. 2021, 11 (37), 22544–22555. 10.1039/D1RA03333F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann C. L.; Streifel B. C.; Lundin J. G.; Wynne J. H. Multifunctional PolyHIPE Wound Dressings for the Treatment of Severe Limb Trauma. Polymer (Guildf) 2017, 126, 408–418. 10.1016/j.polymer.2017.05.067. [DOI] [Google Scholar]
- Onder O. C.; Utroša P.; Caserman S.; Podobnik M.; Žnidarič M. T.; Grdadolnik J.; Kovačič S.; Žagar E.; Pahovnik D. Emulsion-Templated Synthetic Polypeptide Scaffolds Prepared by Ring-Opening Polymerization of: N -Carboxyanhydrides. Polym. Chem. 2020, 11 (26), 4260–4270. 10.1039/D0PY00387E. [DOI] [Google Scholar]
- Onder O. C.; Utroša P.; Caserman S.; Podobnik M.; Žagar E.; Pahovnik D. Preparation of Synthetic Polypeptide-PolyHIPE Hydrogels with Stimuli-Responsive Behavior. Macromolecules 2021, 54 (18), 8321–8330. 10.1021/acs.macromol.1c01490. [DOI] [Google Scholar]
- He X.; Liu J.; Li Z.; Samchek M.; Gates I.; Hu J.; Lu Q. Aqueous Condition-Tolerated High Internal Phase Oil-in-Water Pickering Emulsion as Building Block for Engineering 3D Functional Materials. Chemical Engineering Journal 2022, 446, 137162. 10.1016/j.cej.2022.137162. [DOI] [Google Scholar]
- Wang L. S.; Gopalakrishnan S.; Gupta A.; Banerjee R.; Lee Y. W.; Rotello V. M. Porous Polymerized High Internal Phase Emulsions Prepared Using Proteins and Essential Oils for Antimicrobial Applications. Langmuir 2022, 38 (38), 11675–11682. 10.1021/acs.langmuir.2c01565. [DOI] [PubMed] [Google Scholar]
- Nofar M.; Utz J.; Geis N.; Altstädt V.; Ruckdäschel H. Foam 3D Printing of Thermoplastics: A Symbiosis of Additive Manufacturing and Foaming Technology. Advanced Science 2022, 9 (11), 2105701. 10.1002/advs.202105701. [DOI] [PMC free article] [PubMed] [Google Scholar]