Fig. 1.
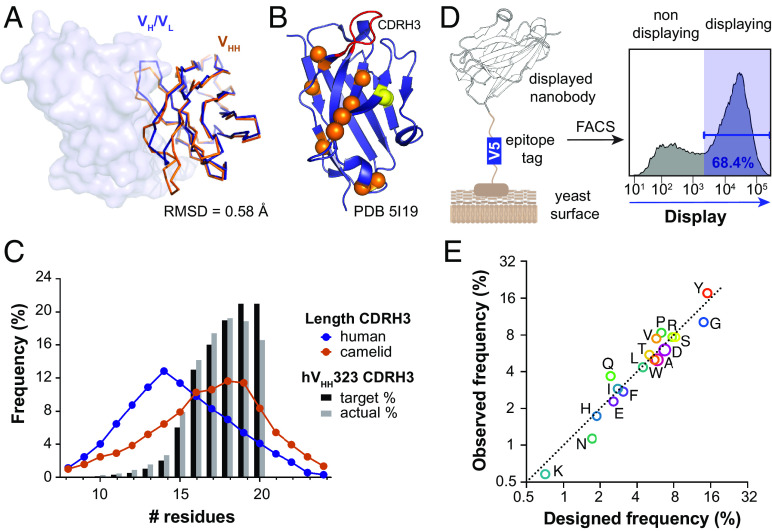
Properties of the synthetic human-based hVHH323 library. (A) Overlay of the VH domain from a conventional human IgG (PDB 5I19, blue) with a representative camel VHH (PDB 5U65, orange). Structures were aligned on the VHH, and the rmsd of the alpha carbons is indicated. The VL domain is shown as surface. (B) VH domain from a human antibody that utilizes VH3-23 (PDB 5I19) with the positions of the mutations that were introduced to produce the hVHH323 scaffold highlighted as spheres. Orange spheres are “camelizing” mutations to remove the need for LC pairing. Yellow spheres indicate the additional disulfide bond that was introduced to increase stability. The variable CDRH3 is colored in red. (C) CDRH3 loop length distribution from the transformed hVHH323 library obtained by deep sequencing analysis compared to human (conventional antibodies) (22) and camelids (HC-only antibodies) (23) CDRH3 repertoires. (D) Schematic yeast display of the hVHH323 nanobodies with a V5 epitope tag. (E) Deep sequencing analysis of AA frequencies of diversified positions in CDRH3s, showing both the intended frequencies and those observed in the transformed library.